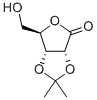
| Identification | More | [Name]
2,3-O-Isopropylidene-D-ribonic gamma-lactone | [CAS]
30725-00-9 | [Synonyms]
2,3-ISOPROPYLIDENE-D-RIBONO-1,4-LACTONE
2,3-ISOPROPYLIDENE-D-RIBONOLACTONE
2,3-O-ISOPROPYLIDENE-D-RIBONIC ACID GAMMA LACTONE
2,3-O-ISOPROPYLIDENE-D-RIBONIC GAMMA-LACTONE
2,3-O-ISOPROPYLIDENE-D-RIBONO-1,4-LACTONE
ISOPROPYLIDENE-D-RIBONOLACTONE
2,3-o-isopropylidene-d-ribonic γ-lactone
D-Ribonic acid, 2,3-O-(1-methylethylidene)-, .gamma.-lactone
D-(2,3-Isopropylidene)lyxono-1,4-lactone
(3aR,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyldihydrofuro[3,4-d][1,3]dioxol-4(3aH)-one | [Molecular Formula]
C8H12O5 | [MDL Number]
MFCD00080793 | [Molecular Weight]
188.18 | [MOL File]
30725-00-9.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
135-139 °C
| [alpha ]
-68.5 º (c=2, Pyridine) | [Boiling point ]
243.17°C (rough estimate) | [density ]
1.2069 (rough estimate) | [refractive index ]
1.4348 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Soluble in methanol at 50mg/ml, acetone, dichloromethane, ethyl Acetate. | [form ]
Powder | [pka]
13.68±0.10(Predicted) | [color ]
White to Off-white | [optical activity]
[α]20/D 69°, c = 2 in pyridine | [Water Solubility ]
soluble | [BRN ]
10090 | [CAS DataBase Reference]
30725-00-9(CAS DataBase Reference) |
| Safety Data | Back Directory | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [HS Code ]
29322090 |
| Hazard Information | Back Directory | [Description]
2,3-Isopropylidene-D-ribonolactone is a building block.1 It has been used in the stereocontrolled synthesis of the carbohydrates 6-epi-trehazolin and 6-epi-trehalamine. | [Chemical Properties]
White Crystalline Solid | [Uses]
2,3-O-Isopropylidene-D-ribonic acid-1,4-lactone is used for the synthesis of C-nucleosides, prostanoids and GABA analogs via homochiral 2-piperidones. It is the starting material for the preparation of biochemical compounds including C-nucleosides, such as neplanocin A, and GABA analogs, via homochiral 2-piperidones. |
|
|