| Identification | More | [Name]
Carbamazepine | [CAS]
298-46-4 | [Synonyms]
5-carbamoyl-5h-dibenz[b,f]azepine
5H-DIBENZ[B,F]AZEPINE-5-CARBOXAMIDE
ANBROMINE
CARBAMAZEPIN
CARBAMAZEPINE
TEGRETOL
5-Carbamoyl-5H-dibenzo(b,f)azepine
5-Carbamoyldibenzo(b,f)azepine
5-Carbamyl-5H-dibenzo(b,f)azepine
5-Carbamyldibenzo(b,f)azepine
5-Carbomoyl-5H-dibenzo(b,f)azepine
5H-Dibenz[b,f]azepine-5-carboxamine
5H-Dibenzo[b,f]azepine-5-carboxamide
Biston
Calepsin
Carbamazepen
Carbamezepine
Carbazepine
Carbelan
Epitol | [EINECS(EC#)]
206-062-7 | [Molecular Formula]
C15H12N2O | [MDL Number]
MFCD00005073 | [Molecular Weight]
236.27 | [MOL File]
298-46-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White Solid | [Melting point ]
191-192 °C (lit.) | [Boiling point ]
378.73°C (rough estimate) | [density ]
1.1099 (rough estimate) | [refractive index ]
1.5906 (estimate) | [Fp ]
9℃ | [storage temp. ]
2-8°C | [solubility ]
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: soluble29mg/mL | [form ]
Crystals | [pka]
13.94±0.20(Predicted) | [color ]
Almost white | [Water Solubility ]
pract. insoluble | [Usage]
Used in treatment of pain associated with trigeminal neuralgia. Anticonvulsant | [Merck ]
1781 | [BCS Class]
2 | [LogP]
2.450 | [CAS DataBase Reference]
298-46-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Carbamazepine(298-46-4) | [EPA Substance Registry System]
5H-Dibenz[b,f]azepine-5-carboxamide (298-46-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R42/43:May cause sensitization by inhalation and skin contact .
R22:Harmful if swallowed.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S37:Wear suitable gloves .
S24:Avoid contact with skin .
S22:Do not breathe dust .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S36:Wear suitable protective clothing . | [RIDADR ]
UN1230 - class 3 - PG 2 - Methanol, solution | [WGK Germany ]
2 | [RTECS ]
HN8225000 | [HS Code ]
29339900 | [Hazardous Substances Data]
298-46-4(Hazardous Substances Data) | [Toxicity]
LD50 orally in mice, rats: 3750, 4025 mg/kg (Stenger, Roulet) |
| Questions And Answer | Back Directory | [Overview]
Carbamazepine (CBZ) is an irninostilbene derivative, structurally similar to the tricyclic antidepressants. Chemically. CBZ is a neutral, liposoluble compound that can easily pass the bloodlbrain barrier and other membranes in the body. Developed and marketed for the treatment of epileptic seizures and trigeminal neuralgia, CBZ has been utilized more and more frequently in the last decade to treat psychiatric disorders.
Carbamazepine was first evaluated as a potential treatment in manic depressive psychosis by Takezaki and Hanaoka (1971)[1], and Okuma et al. (1973)[2]. In 1979, Okuma et al.[3] performed the first double-blind trial of carbamazepine in comparison with the antipsychotic chlorpromazine in mania and found that 70% and 60% of patients improved respectively. A further placebo-controlled double-blind study replicated the antimanic properties of carbamazepine under controlled circumstances (Ballenger and Post, 1980). There have since been many studies demonstrating the efficacy of carbamazepine in treating the acute manic and depressive symptoms of bipolar disorder, as well as in prophylaxis[4, 5].
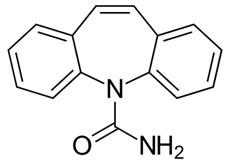
Figure 1 the chemical structure of carbamazepine
Carbamazepine is a first- generation antiepileptic drug (AED) known with the proprietary brand name of Tegretol® (Novartis, Basel) in the UK and USA (Fig. 3.2). Oxcarbazepine is a second- generation AED supplied under the proprietary brand name of Trileptal® (Novartis, Basel) in the UK and USA (Fig. 3.3). Eslicarbazepine is a third- generation AED sold under the proprietary brand names of Zebinix® (Eisai, Hatfield) in the UK and Aptiom® (Sunovion, Marlborough, MS) in the USA. | [Indication]
Carbamazepine has been used to treat many types of epilepsies since the early sixties[6]. The psychotropic properties of the drug were soon recognized and prompted studies in psychiatric disorders. Today, the main indications for CBZ in psychiatry are the treatment of acute manic states and the prophylaxis of recurrence in bipolar disorders[7]. The clinical studies reviewed consistently reported a CBZ success rate in acute manic states and recurrence prophylaxis in bipolar disorders ranging from 50 to 65%. More recent works confirmed the success rate in acute manic states[8], whereas data for the long-term prophylaxis of recurrence were more variable, and in general less positive[9]. Post et al. (1993) [10] suggested that poor long-term CBZ efficacy might be due to tolerance development and, on the basis of the CBZ action in experimental models. Speculated that periods of drug "holiday" may restore its efficacy. A similar warning has been reported for the long-term prophylactic efficacy of lithium[11].
Evidence of some positive action of CBZ in unipolar depression has also been reported[12]. More recently. Cullen et al. (1991) [13] retrospectively analyzed 16 melancholic patients who received CBZ alone or in combination with other psychotropic drugs. Seven patients had a moderate to marked improvement, but treatment had to be discontinued in five due to side effects. Stuppaeck et al. (1993) [14], in open observations in 15 patients with unipolar depression, concluded that CBZ had a positive effect in 11 patients.
Other psychiatric syndromes for which CBZ has been proved or suggested to be useful include aggressive agitation in demented patients[15]. Super-sensitivity psychosis[16], and alcohol[17] and benzodiazepine withdrawal syndromes[18]. In panic disorders, CBZ has been reported to attenuate panic attacks in subjects with underlying EEG abnormalities[19]. However, in the only controlled trial in 14 patients with panic disorders, CBZ was no better than placebo[20].
Recommendations
- Seizure types: first line (generalized tonic- clonic seizures, focal seizures), adjunctive (focal seizures), contraindicated (generalized tonic- clonic seizures if there are absence or myoclonic seizures, or if juvenile myoclonic epilepsy is suspected, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types: first line (epilepsy with generalized tonic- clonic seizures only, benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, late- onset childhood occipital epilepsy), adjunctive (benign epilepsy with centrotemporal spikes, Panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, juvenile myoclonic epilepsy, idiopathic generalized epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
- Psychiatry: prophylaxis of manic- depressive phases in patients with bipolar disorder unresponsive to lithium therapy; treatment of alcohol withdrawal symptoms (unlicensed).
- Neurology: treatment of paroxysmal pain in trigeminal neuralgia and diabetic neuropathy (unlicensed).
| [Dosage and administration]
Carbamazepine dosages employed in the various pathologies are similar: in most cases therapeutic effects are obtained at doses of 10 to 20 mg/kg/day. Age has only a limited effect on CBZ disposition, but children may need a slightly higher dosage (in mg/kg). No dosage adjustments are required in relation to patient's sex or other genetic factors. Treatment should be started at low dosages (3 - 5 mg/kg/day) and increased progressively by a similar amount every 5-7 days, until the desired clinical effect is obtained or persistent side effects appear. This approach will determine the lowest effective (or the maximum tolerated) dose and reduce the incidence of dose-dependent side effects, which are more frequent and more troublesome for the patient at the beginning of therapy[15]. If tolerance is poor but the drug seems to be clinically effective, an even slower titration may prove useful. Treatment of acute manic states, however, may require a faster titration at the cost of an increased frequency of side effects[16]. A daily dose administered in conventional tablets or oral suspension should be divided into 3-4 intakes to avoid excessive fluctuations of plasma concentration; alternatively, slow-release preparations can be used. In some countries a chewable tablet formulation (100 mg) is available, which may be convenient in children[13].
- Epilepsy—immediate release: 100– 200 mg od/ bd, increased by 100– 200 mg every 14 days; usual maintenance 800– 1200 mg daily, in divided doses (max. 2000 mg daily).
- Epilepsy— prolonged release: 50– 200 mg bd, increased by 100– 200 mg every 14 days; usual maintenance 800– 1200 mg daily, divided into two doses (max. 2000 mg daily).
- Bipolar disorder— immediate release: 400 mg daily, in divided doses, increased by 100– 200 mg every 14 days; usual maintenance 400– 600 mg daily, in divided doses (max. 1600 mg daily).
- Bipolar disorder—prolonged release: 200 mg bd, increased by 100– 200 mg every 14 days; usual maintenance 200– 300 mg bd (max. 1600 mg daily).
If stopping carbamazepine, patients with bipolar disorder need to reduce the dose gradually over a period of at least 4 weeks.
| [Plasma levels monitoring]
Correlations between dosages and plasma levels of carbamazepine, as well as between plasma levels, and clinical efficacy or tolerability, are rather tenuous. However, monitoring of the plasma levels (therapeutic range in the treatment of epilepsy 4–12 mg/ L) may be useful in selected conditions, such as a dramatic increase in seizure frequency/ verification of patient compliance, during pregnancy, in suspected absorption disorders, in suspected toxicity due to polymedication.
| [Cautions]
- Patients with a history of hepatic porphyrias.
- Patients with a history of bone marrow depression.
- Patients with atrioventricular block.
- Patients with a history of haematological reactions to other drugs.
- Patients with susceptibility to angle- closure glaucoma.
- Patients with skin reactions.
- Patients with cardiac disease.
- Patients with absence and myoclonic seizures.
| [Pharmacodynamics]
Carbamazepine given in conventional tablets shows a mean peak concentration time during chronic treatment of about 4 hours, with an estimated oral bioavailability of 80-90%[21]. Carbamazepine is readily distributed in the body with an apparent volume of distribution (estimated on the presumption of a 100% oral bioavailability) of 0.8 2.0 /kg. Brain concentrations are about 1.21.4 times those in plasma and the breast milk/ total plasma level ratio is about 0.4 [22]. In plasma, CBZ binds to circulating proteins, mostly albumin and a1-acid glycoprotein. The bound fraction is about 70-80% and is constant in the interval of plasma concentrations normally observed in therapy[23].
Carbamazepine is mainly eliminated through hepatic metabolism by microsomal enzymes of the cytochrome P450 family. The main metabolites are epoxy-CBZ and 10.11-dihydro, 10,lldihydroxy carbarnazepine (CBZ-DIOL). In man, the hydroxylated derivatives are conjugated with glucuronic acid and excreted by the kidneys. Small amounts of unmodified CBZ are found in feces and in urine[24]. Quantitatively, the main urinary metabolite is CBZ-DIOL, which is, however, inactive. Carbamazepine-l0, 11-epoxideh as anticonvulsant potency similar to the parent drug, and has comparable efficacy in trigerninal neuralgia in man. After repeated dose, CBZ induces its own metabolism and its half-life is reduced by about 50%. Changing, for example, from 36 hours at the beginning of therapy to 21 hours after 3 weeks of treatment. Patients treated with other enzyme-inducing drugs may have a CBZ half-life as short as 5 16 hours. Children present a slightly higher CBZ clearance[21].
| [Interactions]
With AEDs
- Plasma concentration of carbamazapine is increased by vigabatrin, whereas plasma concentration of the active metabolite carbamazepine 10,11- epoxide is increased by primidone and valproate (reduce carbamazepine dose to avoid increased risk of toxicity).
- Plasma concentration of carbamazapine is reduced by cytochrome P450 3A4 inducers (including eslicarbazepine, oxcarbazepine, phenobarbital, phenytoin, primidone, and, possibly, clonazepam).
- Carbamazapine is a cytochrome P450 3A4 inducer and can decrease the plasma concentration of clobazam, clonazepam, ethosuximide, lamotrigine, oxcarbazepine, primidone, tiagabine, topiramate, valproate, and zonisamide.
- Co- administration of levetiracetam has been reported to increase carbamazepine- induced toxicity; cross- sensitivity has been reported with oxcarbazepine and phenytoin.
With other drugs
- Plasma concentration of carbamazapine is reduced by cytochrome P450 3A4 inducers aminophylline, cisplatin, doxorubicin, St John wort (Hypericum perforatum), isotretinoin, rifampicin, and theophylline (consider increasing the dose of carbamazapine).
- Plasma concentration of carbamazapine is increased by cytochrome P450 3A4 inhibitors: acetazolamide, azoles (antifungals), cimetidine, ciprofloxacine, danazol, dextropropoxyphene, diltiazem, fluoxetine, fluvoxamine, isoniazid, loratadine, macrolide antibiotics, olanzapine, omeprazole, paroxetine, protease inhibitors (antivirals), trazodone, and verapamil. Plasma concentration of the active metabolite carbamazepine- 10,11- epoxide is increased by progabide, quetiapine, valnoctamide, and valpromide.
- Carbamazepine is a cytochrome P450 3A4 inducer and can decrease the plasma concentration of albendazole, alprazolam, aprepitant, aripiprazole,atorvastatin, bromperidol, buprenorphine, bupropion, calcium channel blockers (e.g. felodipine), cerivastatin, ciclosporin, citalopram, clozapine, corticosteroids (e.g. prednisolone, dexamethasone), cyclophosphamide, digoxin, doxycycline, everolimus, haloperidol, hormonal contraceptives (oestrogens and progesterones), imatinib, itraconazole, ivabradine, lapatinib, levothyroxine, lovastatin, methadone, mianserin, olanzapine, oral anticoagulants (e.g. warfarin), paliperidone, paracetamol (acetaminophen), protease inhibitors (antivirals), quetiapine, rifabutin, risperidone, sertraline, simvastatin, tacrolimus, tadalafil, temsirolimus, theophylline, tramadol, trazodone, tricyclic antidepressants, voriconazole, and sirolimus.
With alcohol/food
- Drinking alcohol may affect patients more than usual; eating grapefruit, or drinking grapefruit juice, may increase chance of experiencing adverse effects.
| [Special populations]
Hepatic impairment
- Metabolism impaired in advanced liver disease.
Renal impairment
Pregnancy
- Developmental disorders and malformations (including spina bifida), as well as other congenital anomalies (including craniofacial defects, such as cleft lip/ palate, cardiovascular malformations, hypospadias, and anomalies involving various body systems) have been reported in association with the use of carbamazepine during pregnancy. In women of childbearing age carbamazepine should, wherever possible, be prescribed as monotherapy, because the incidence of congenital abnormalities in the offspring of women treated with a combination of antiepileptic drugs is greater (especially if valproate is part of the polytherapy).
- Pregnant women with epilepsy should be treated with minimum effective doses of carbamazepine and monitoring of plasma levels is recommended (aiming at the lower side of the therapeutic range, as there is evidence to suggest that the risk of malformation with carbamazepine may be dose- dependent).
- Should a woman on carbamazepine decide to breastfeed, the infant should be monitored for possible adverse effects, as carbamazepine can be excreted in considerable amounts in breastmilk, which in combination with slow infantile elimination can result in plasma concentrations at which pharmacological effects occur. Since there have been reports of cholestatic hepatitis in neonates exposed to carbamazepine during antenatal and or during breastfeeding, breastfed infants of mothers treated with carbamazepine should be carefully observed for adverse hepatobiliary effects.
| [Mode of action]
The many effects of CBZ on the central nervous system include: the ability to bind to neuron membrane sodium channels when they are in the inactivated state, slowing the speed of reactivation and thus reducing the neuron's capacity of high frequency firing[25]; selective interaction with adenosine receptors and modification of the activity of second messengers like CAMP and cGMP and modification of various neurotransmitter systems[26]. An interesting aspect of CBZ action on the brain is the apparent selectivity for the limbic system, a feature that may provide an anatomical basis for both the psychotropic action of the drug and its efficacy in psychomotor epilepsies.
Carbamazepine is characterized by a good behavioural and cognitive profile in patients with epilepsy. Overall, adverse psychiatric effects (especially irritation, agitation, depression) are rarely reported in this patient population. Moderate cognitive problems affecting attention, memory, and language have occasionally been reported (especially at high doses).
| [Psychiatric use]
Carbamazepine was approved as a treatment for acute mania in 2004, decades after it was recognized as an effective alternative to lithium in the management of bipolar illness. Data from open- label trials suggest that carbamazepine is effective in the prophylaxis of bipolar disorder or acute mania, but may be less effective than valproate or lithium. However, carbamazepine has been suggested to be a better alternative for atypical manifestations of bipolar disorder, such as rapid cycling course, mood- incongruent delusions, or in the presence of other co- morbid psychiatric or neurological conditions. Carbamazepine may also be effective in unipolar depression, whereas its utility in schizophrenia is uncertain. In rarer cases, carbamazepine has been used to treat aggressive behaviour and to facilitate sedatives/ alcohol withdrawal, but there is no solid evidence to date to establish its efficacy in this domain. To summarize, evidence is strongest to support the mood stabilizing properties of carbamazepine.
| [Adverse reactions and precaution]
The most common manifestations of acute CBZ toxicity at therapeutic dosages involve the central nervous and gastrointestinal systems (sedation. nystagmus, diplopia, ataxia, dizziness, nausea, vomiting, constipation, diarrhea) [27]. Dry mouth may occur as a consequence of some anticholinergic action of the drug. These symptoms respond to a dose reduction and, due to tolerance development, usually abate during treatment. Carbamazepine may induce involuntary movements such as myoclonic and choreoathetoid jerks, dystonia and asterixis[28]. Carbarnazepine has negative chmnotropic and dromotropic effects on cardiac conduction and may induce various types of bradyarrhythmia or even a complete atrioventricular block[28]. These dangerous complications are usually observed in elderly patients or patients with a preexisting impairment of cardiac conduction, are concentration-related, and require dose reduction or drug suspension.
During pregnancy, important modifications of CBZ kinetics may occur, requiring plasma concentration monitoring and, possibly, dosage adjustment[22]. CBZ enters breast milk (milk/plasma ratio of about 0.4)and a transfer of 1-4 mg/day of drug from mother to child takes place during nursing[22]. Concentrations in the breast-fed infant should not normally exceed 0.5 1.0 ug/ml, but somewhat higher concentrations, up to 4.7 ~ug /ml one case, have occasionally been reported[22]. These findings suggest that breast-feeding is not to be discouraged and that it should be discontinued only if the newborn shows signs of distress, such as blunted suck reflex.
| [References]
- Takezaki H, Hanaoka M (1971). The use of carbamazepine (Tegretol) in the control of manic-depressive psychosis and other manic-depressive states. Clinical Psychiatry 13, 173–183.
- Okuma T, Kishimoto A, Inoue K, Matsumoto H, Ogura A, Matsushita T, Nakao T, Ogura C (1973). Anti-manic and prophylactic effects of carbamazepine (Tegretol) on manic-depressive psychosis: a preliminary report. Folia Psychiatrica et Neurologica 27, 283–297.
- Okuma T, Inanaga K, Otsuki S, Sarai K, Takahashi R, Hazama H, Mori A, Watanabe M (1979). Comparison of the antimanic efficacy of carbamazepine and chlorpromazine : a double-blind controlled study. Psychopharmacology 66, 211–217.
- Brambilla P, Barale F, Soares JC (2001). Perspectives on the use of anticonvulsants in the treatment of bipolar disorder. International Journal of Neuropsychopharmacology 4, 421–446.
- Post RM, Ketter TA, Denicoff K (1996a). The place of anticonvulsant therapy in bipolar illness. Psychopharmacology 128, 115–129.
- Loiseau. P, B. Duche: Carbamazepine. Clinical use. In: Levy, R H., E E. Dreifus. R H. Mattson, B. S. Me1drum.J. K. Penly (eds.): Antiepileptic drugs. Raven Press. New York 7989 p. 553-554
- Michels. R. P. M. Marzuk: Progress in psychiatry. N. Engl. J. Med. 329 (1993) 628 -638
- Okuma, 1, I. Yamashita, R Takahashi, H. Itoh, S. Otsuki. S. Watanabe. S. Sarai. H. Hazama. K. Inanaga: Comparison of the antimanic efficacy of carbamazepine and lithium carbonate by doubleblind controlled study. Pharmacopsychiatry 23 (1990) 143-150
- Small, J. G., M. H. Klapper, V. Milstein.].]. Kellmans, M.J. Miller.]. D. Marhenke, I. E Small: Carbamazepine compared with lithium in the treatment of mania. Arch. Gen. Psych. 48 (1991) 915-921
- Post, R M., 1 A. Ketter, P. J. Pauaglia. M. S. George, L. MamngelI, K. Denicoff: New developments in the use of anticonvulsants as mood stabilizers. Neuropsychobiology 27 (1993) 132-137
- Maj, M., R Pimzzi. D. Kemali: Long-term outcome of lithium prophylaxis in patients initially classified as complete responders. Psychopharmacology 98 (1989) 535-538
- Evans, R W., C. T. Gualtieri: Carbamazepine: a neurological and psychiatric profile. Clin. Neuropharrnacol. 8 (1985) 221 -241
- Cullen. M., P. Mitchell, H. Brodaty, I? Royce, G. Parker, I. Hickie. K Wilhelm: Carbamazepine for treatment-resistant melancholia. J. Clin. Psychiatry 52 (1991) 472-476
- Stuppaeck, C. H., C H. Barnas, K. Hackenberg, C. H. Miller. W. W. Neischhacker: Carbamazepine monotherapy in the treatment of alcohol withdrawal. Int. Clin. Psychopharmacol. 5 (1990)2 73 -278
- Gleason, R P, L, S. Schneider: Carbamazepine treatment of agitation in Alzheimer's outpatients refractory to neuroleptics. J. Clin. Psychiatry 51 (1990) 115118
- Chouinard G.. S. Sultan: Treatment of supersensitivity psychosis with antiepileptic drugs: report of a series of 43 cases. Psychopharmacol. Bull. 26 (1990) 337-341
- Stuppaeck, C. H., C H. Barnas, K. Hackenberg, C. H. Miller. W. W. Neischhacker: Carbamazepine monotherapy in the treatment of alcohol withdrawal. Int. Clin. Psychopharmacol. 5 (1990)2 73 -278
- Garcia. B.. E. Zaborras, V. Arease. G. Obeso, I. jimena, P. DeJuana, T. Bermejo: Interaction between isoniazid and carbamazepine potentiated by cimetidine. DlCP 26 (1992) 841
- Keck, F. E. jr, S. L McElroy. K. C. Tugrul. J. A. Bennett,. M. R. Smith: Antiepileptic drugs for the treatment of panic disorder. Neurobiology 27 (1993) 150-153
- Uhde, Z W.. M. B. Stein, R. M. Post: Lack of efficacy of carbamazepine in the treatment of panic disorder. Am. J. Psychiatry 145 (1988) 1104-1109
- Morselli, P. L: Carbamazepine. Absorption, distribution and excretion. In: Levy, R H., E E Dreifuss, R H. Mattson, B. S. Me1drum.J. K. Penry (eds.): Antiepileptic Drugs. Raven Press, New York 1989 p. 473-490
- Nau. H., W. Ku hnz, H. J. Egger. D. Rating, H. Helge: Anticonvulsants during pregnancy and lactation. Transplacental, maternal and neonatal pharmacokinetics. Clin. Pharmacokinet. 7 (1982) 508543
- Contin, M., R Riva, E Albani. E. Perucca. G. Lamonfanara, A. Banrui: a1-acid glycoprotein concentration and serum protein binding of carbamazepine and carbamazepine 10,ll-epoxide in children with epilepsy. Eur. J. Clin. Pharmacol. 29 (1985) 211 -214
- Faigle.J. W., K. E Feldmann: Carbamazepine Biotransformation In: Levy. R. H.. E E. Dreifuss, R H. Mattson, B. 5. Me1dnrm.j. K. Penry (eds.): Antiepileptic Drugs. Raven Press, New York 1989, p.491 – 504
- Macdonald, R. L: Carbamazepine. Mechanisms of action. In: Levy, R H.. E. Dreifiss, R H. Mattson. B. S. Meldrum,]. K. Penry (eds.): Antiepileptic drugs. Raven Press. New York 1989, p. 447-471
- Motohashi. N.: Gaba receptor alterations after chronic lithium administration. Comparison with carbamazepine and sodium valproate. Prog. Neuro-psychophannacol. Biol. Psychiatry 16 (1992) 571 – 579
- Askrnark, H.. B. E. Wiholm: Epidemiology of adverse reactions to carbamazepine as seen in a spontaneous reporting system. Acta Neurol. Scand. 81 (1990) 131 -140
- Gram L, P K. jensen: Carbarnazepine Toxicity. In: Levy. R. H., R. Mattson, B. Meldrum, J. K Pen% E E. Dreifuss (eds.): Antiepileptic Drugs. Raven Press. New York 1989, p. 555-565
|
| Hazard Information | Back Directory | [Description]
Carbamazepine is a synthetic iminostilbene derivative structurally
similar to imipramine, a tricyclic antidepressant. While
unrelated structurally, carbamazepine shares a similar therapeutic
action with phenytoin. Carbamazepine was first discovered
in 1953 by Swiss chemist Walter Schindler. Throughout the
1960s, antimuscarinic was used and marketed for trigeminal
neuralgia and as an anticonvulsant. By the 1970s, it was being
used as a mood stabilizer for patients with bipolar disorder. | [Chemical Properties]
White Solid | [Originator]
Tegretol,Geigy,W. Germany,1964 | [Uses]
analgesic, anticonvulsant | [Uses]
Carbamazepine (CBZ) is a first generation anticonvulsant and mood stabilizing compound that has been used as a therapeutic in the context of neuropathic pain, epilepsy, and affective disorders. It exerts its effects by blocking voltage-gated sodium channels (IC50 = 640 μM), making fewer of these channels available to subsequently open, which leads to decreased high-frequency repetitive firing of action potentials. The estimated IC50 values for inhibition of Nav1.7-, Nav1.3-, and Nav1.8-type channels by CBZ following prolonged inactivation have been reported as 406, 900, and 138 μM, respectively. CBZ can also inhibit L-type Ca2+ channels (IC50 = 974 μM) and has been shown to potentiate GABAA receptors (IC50 >3 mM). | [Uses]
Used in treatment of pain associated with trigeminal neuralgia. Anticonvulsant | [Definition]
ChEBI: A dibenzoazepine that is 5H-dibenzo[b,f]azepine carrying a carbamoyl substituent at the azepine nitrogen, used as an anticonvulsant. | [Manufacturing Process]
19.3 parts of iminostilbene are dispersed in 100 parts by volume of toluene.
Phosgene is then introduced whereupon the temperature of the reaction
mixture rises to 70°C. While boiling under reflux, further phosgene is
introduced until all the iminostilbene has dissolved and the hydrogen chloride
development is complete. The reaction mixture is then cooled and the 5-
chlorocarbonyl iminostilbene which has crystallized out is filtered off under
suction. It melts at 168° to 169°C.
12.8 parts of 5-chlorocarbonyl iminostilbene are dispersed in 128 parts by
volume of absolute ethanol and ammonia gas is introduced for three hours
into this mixture while stirring at boiling temperature. The reaction is
complete after this time; the reaction mixture is cooled and the crystals which
precipitate are filtered off under suction. The ammonium chloride is washed
from the crystals with water and the residue is recrystallized first from
absolute ethanol and then from benzene. 5-carbamyl iminostilbene is obtained
which melts at 204° to 206°C. | [Brand name]
Carbatrol (Shire); Epitol (Teva); Equetro (Shire); Tegretol
(Novartis); Teril (Taro). | [Therapeutic Function]
Analgesic, Anticonvulsant | [Biological Functions]
Carbamazepine has become a major drug in the treatment
of seizure disorders. It has high efficacy, is well tolerated
by most patients, and exhibits fewer long-term
side effects than other drugs.
Oral absorption of carbamazepine is quite slow and
often erratic. Its half-life is reported to vary from 12 to
60 hours in humans.The development of blood level assays
has markedly improved the success of therapy with
this drug, since serum concentration is only partially
dose related. Carbamazepine is metabolized in the liver,
and there is evidence that its continued administration
leads to hepatic enzyme induction. Carbamazepine-
10,11-epoxide is a pharmacologically active metabolite with significant anticonvulsant effects of its own. | [General Description]
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.
Carbamazepine is a tricyclic lipophilic compound, with mild anticholinergic activity. It is widely used as an antiepileptic drug for the treatment of simple and complex partial tonic-clonic seizures. | [Biochem/physiol Actions]
Anticonvulsant; ligand for the GABAA receptor benzodiazepine modulatory site. Sodium channel inhibitor. | [Mechanism of action]
In animals, the profile of antiseizure properties for CBZ is similar to that of phenytoin. CBZ is effective in the maximal
electroshock (MES) test (electrically induced seizure test) but is ineffective against pentylenetetrazole-induced seizures. It is
not effective for absence or myoclonic seizures and, indeed, may exacerbate their onset. Like phenytoin, CBZ acts on
voltage-dependent sodium channels to prevent the spread of seizures. CBZ depresses synaptic transmission in the reticular
activating system, thalamus, and limbic structures. In a double-blind, crossover study in patients whose seizures were not
controlled completely by combinations of AED, CBZ was equal in efficacy to phenobarbital and phenytoin in controlling seizure
frequency, and side effects were minimal. | [Pharmacokinetics]
Following the administration of an oral dose, CBZ is slowly absorbed, with the attainment of peak concentration from
immediate-release tablets in 4 to 5 hours and from extended-release tablets in 3 to 12 hours. The normal half-life averages
between 12 and 17 hours; however, because of autoinduction, the half-life may range from 8 to 29 hours. The half-life for
CBZ-10,11-epoxide is 5 to 8 hours. Therapeutic plasma concentrations range from 4 to 12 μg/mL (in adults) and may require a
month to achieve a stable therapeutic concentration for the desired antiseizure effect because of induction of hepatic
metabolizing enzymes.
CBZ is principally metabolized by CYP3A4 its 10,11-epoxide, with CYP2C8 and CYP1A2 having minor roles. CBZ epoxide is
hydrolyzed to inactive 10,11-dihydroxy CBZ by epoxide hydrolase. CBZ epoxide is active and appears to be more toxic than
CBZ. However, CBZ not only induces CYP3A4 activity but also its own metabolism (an autoinducer) as well as UGT and
the increased formation of glucuronide metabolites. Like phenytoin, CBZ has been associated with a number of toxic effects,
including a drug-induced hypersensitivity syndrome. Although phenytoin-induced hypersensitivity reactions are relatively rare
events, they can be potentially life-threatening. Although the mechanism by which CBZ induces hypersensitivity reactions has
not been well characterized, recent studies have suggested that the immune reaction may be caused, at least in part, by its
metabolism into chemically reactive metabolites, which may be the critical step in the formation of protein adducts and
subsequent immune responses. | [Clinical Use]
Carbamazepine is an effective agent for the treatment
of partial seizures and generalized tonic–clonic
seizures; its use is contraindicated in absence epilepsy.
Carbamazepine is also useful in the treatment of
trigeminal neuralgia and is an effective agent for the
treatment of bipolar disorders. | [Side effects]
Like most of the agents that block sodium channels,
side effects associated with carbamazepine administration
involve the central nervous system (CNS).
Drowsiness is the most common side effect, followed by
nausea, headache, dizziness, incoordination, vertigo, and
diplopia.These effects occur particularly when the drug
is first taken, but tolerance often develops over a few
weeks. There appears to be little risk of cognitive impairment
with carbamazepine.
Carbamazepine causes a variety of rashes and other
allergic reactions including fever, hepatosplenomegaly,
and lymphadenopathy, but the incidence of serious hypersensitivity
reactions is rare. Systemic lupus erythematosus
can occur, but discontinuation of the drug
leads to eventual disappearance of the symptoms.
Idiosyncratic hematological reactions to carbamazepine may occur, but serious blood dyscrasias are rare.
Carbamazepine has been shown to exacerbate or precipitate
seizures in some patients, particularly those exhibiting
generalized atypical absences.
While the number of side effects may be fairly large,
most are not serious and can be managed. Severe adverse
reactions occur less commonly than with phenytoin
and similar drugs. The overall incidence of toxicity
seems to be fairly low at usual therapeutic doses.
Most of the drug interactions with carbamazepine
are related to its effects on microsomal drug metabolism.
Carbamazepine can induce its own metabolism
(autoinduction) after prolonged administration, decreasing
its clearance rate, half-life, and serum concentrations.
The possibility of autoinduction requires the
clinician to reevaluate the patient’s blood levels after a
month of carbamazepine therapy. The autoinduction
phenomenon is over in about a month.
Carbamazepine also can induce the enzymes that
metabolize other anticonvulsant drugs, including
phenytoin, primidone, phenobarbital, valproic acid,
clonazepam, and ethosuximide, and metabolism of
other drugs the patient may be taking. Similarly, other
drugs may induce metabolism of carbamazepine; the
end result is the same as for autoinduction, and the dose
of carbamazepine must be readjusted. A common
drug–drug interaction is between carbamazepine and
the macrolide antibiotics erythromycin and troleandomycin.
After a few days of antibiotic therapy, symptoms
of carbamazepine toxicity develop; this is readily
reversible if either the antibiotic or carbamazepine is
discontinued. | [Synthesis]
Carbamazepine, 5H-dibenz[b,f]azepine-5-carboxamide (9.5.2), is syn�thesized by reacting 5H-dibenz[b,f]azepine and phosgene, which forms 5-chlorcarboxy-
5H-dibenz-[b,f]azepine (9.5.1), and its subsequent reaction with ammonia to give the desired carbamazepine (9.5.2) [16]. An alternative method of synthesis is the direct reac�tion of 5H-dibenz[b,f]azepine with potassium cyanate [17].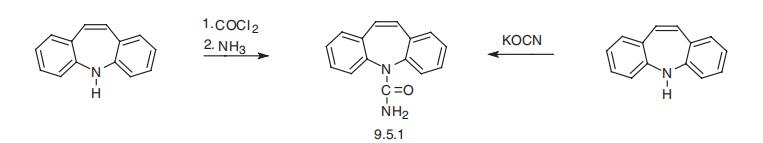
| [Drug interactions]
Potentially hazardous interactions with other drugs
Analgesics: effect enhanced by dextropropoxyphene;
decreased effect of fentanyl, tramadol and
methadone; possibly increases paracetamol
metabolism, also reports of hepatotoxicity.
Anthelmintics: concentration of albendazole and
praziquantel reduced - consider increasing dose for
systemic infections.
Anti-arrhythmics: possibly reduces dronedarone
concentration - avoid.
Antibacterials: reduced effect of doxycycline;
concentration increased by clarithromycin,
erythromycin and isoniazid; increased risk
of isoniazid hepatotoxicity; possibly reduces
bedaquiline concentration - avoid; avoid with
delamanid; concentration reduced by rifabutin;
concentration of telithromycin reduced - avoid.
Anticoagulants: metabolism of coumarins accelerated
(reduced anticoagulant effect); concentration of
apixaban and dabigatran possibly reduced - avoid;
concentration of edoxaban possibly reduced;
concentration of rivaroxaban possibly reduced -
monitor for signs of thrombosis.
Antidepressants: antagonism of anticonvulsant
effect; concentration increased by fluoxetine and
fluvoxamine; concentration of mianserin, mirtazapine,
paroxetine, reboxetine, trazodone, tricyclics
and vortioxetine reduced; avoid with MAOIs;
concentration reduced by St John’s wort - avoid.
Antiepileptics: concentration of eslicarbazepine
possibly reduced but risk of side effects increased;
concentration of ethosuximide, retigabine, topiramateand valproate possibly reduced, concentration
of active carbamazepine metabolite increased by
valproate; concentration of lamotrigine, perampanel,
tiagabine and zonisamide reduced; concentration of
phenobarbital and primidone increased; increased
risk of carbamazepine toxicity with levetiracetam;
concentration sometimes reduced by oxcarbazepine
but active metabolite of carbamazepine may
be increased and oxcarbazepine metabolite
reduced; concentration of both drugs reduced
with fosphenytoin, phenytoin and rufinamide,
fosphenytoin and phenytoin concentration may also
be increased; concentration increased by stripentol.
Antifungals: concentration possibly increased
by fluconazole, ketoconazole and miconazole;
concentration of itraconazole, isavuconazole,
caspofungin, ketoconazole, posaconazole and
voriconazole possibly reduced, avoid with isavuconazole
and voriconazole; consider increasing caspofungin dose.
Antimalarials: avoid with piperaquine with
artenimol; chloroquine, hydroxychloroquine and
mefloquine antagonise anticonvulsant effect.
Antipsychotics: antagonism of anticonvulsant effect;
reduced concentration of aripiprazole (avoid or
increase aripiprazole dose), haloperidol, clozapine,
lurasidone (avoid), olanzapine, paliperidone,
quetiapine and risperidone; avoid concomitant use
with other drugs that can cause agranulocytosis.
Antivirals: concentration of boceprevir, daclatasvir,
dasabuvir, ombitasvir, paritaprevir, rilpivirine
and simeprevir reduced - avoid; possibly
reduced concentration of darunavir, dolutegravir,
fosamprenavir, indinavir, lopinavir, nevirapine,
saquinavir and tipranavir; concentration possibly
increased by indinavir and ritonavir; concentration of
both drugs reduced in combination with efavirenz;
avoid with elvitegravir, etravirine, ledipasvir,
sofosbuvir and telaprevir.
Apremilast: possibly reduces apremilast
concentration - avoid.
Calcium-channel blockers: effects enhanced by
diltiazem and verapamil; reduced effect of felodipine,
isradipine and probably dihydropyridines, nicardipine,
nifedipine and nimodipine - avoid with nimodipine.
Cannabis extract: possibly reduces cannabis extract
concentration - avoid.
Ciclosporin: metabolism accelerated (reduced
ciclosporin concentration).
Clopidogrel: possibly reduced antiplatelet effect.
Cobicistat: possibly reduces cobicistat concentration
- avoid.
Corticosteroids: reduced effect of corticosteroids.
Cytotoxics: possibly reduced concentration of
axitinib, increase axitinib dose; possibly reduced
concentration of bortezomib, bosutinib, ceritinib,
crizotinib, dasatinib, ibrutinib, idelalisib, imatinib,
lapatinib, ponatinib, vandetanib and vismodegib and
possibly cabozantinib - avoid; avoid with cabazitaxel,
dabrafenib, gefitinib, olaparib, panobinostat and
vemurafenib; concentration of irinotecan and its active
metabolite and possibly eribulin reduced; increased
risk of sensitivity reactions with procarbazine.
Diuretics: increased risk of hyponatraemia;
concentration increased by acetazolamide; reduced
eplerenone concentration - avoid.
Fesoterodine: concentration of active metabolite of
fesoterodine reduced - avoid.
Guanfacine: possibly reduces guanfacine
concentration - increase guanfacine dose.
Hormone antagonists: possibly reduces abiraterone
concentration - avoid; metabolism inhibited by
danazol; possibly accelerated metabolism of toremifene.
Ivacaftor: possibly reduces ivacaftor concentration -
avoid.
Lipid-regulating drugs: concentration of simvastatin
reduced.
Naloxegol: possibly reduces naloxegol concentration
- avoid.
Oestrogens and progestogens: reduced contraceptive
effect.
Orlistat: possibly increased risk of convulsions.
Ulcer-healing drugs: concentration increased by
cimetidine.
Ulipristal: contraceptive effect possibly reduced -
avoid. | [Environmental Fate]
Carbamazepine is both an important anticonvulsant in therapeutic
doses and a powerful proconvulsant in overdose. The
therapeutic anticonvulsant mechanism is primarily related to
blockade of presynaptic voltage-gated sodium channels.
Blockade of the sodium channels is believed to inhibit the
release of synaptic glutamate and possibly other neurotransmitters.
Carbamazepine is also a powerful inhibitor of the
muscarinic and nicotinic acetylcholine receptors, N-methyl-Daspartate
(NMDA) receptors, and the central nervous system
(CNS) adenosine receptors. In addition, carbamazepine is
structurally related to the cyclic antidepressant imipramine
and in massive overdose, it may affect cardiac sodium
channels. | [Metabolism]
Carbamazepine is metabolised in the liver by
cytochrome P450 3A4, where the epoxide pathway
of biotransformation yields the 10, 11-transdiol
derivative and its glucuronide as the main metabolites.
9-Hydroxy-methyl-10-carbamoyl acridan is a minor
metabolite related to this pathway. Other important
biotransformation pathways for carbamazepine lead to
various monohydroxylated compounds, as well as to the
N-glucuronide of carbamazepine produced by UGT2B7.
After administration of a single oral dose of 400 mg
carbamazepine, 72% is excreted in the urine and 28% in
the faeces. In the urine, about 2% of the dose is recovered
as unchanged drug and about 1% as the pharmacologically
active 10,11-epoxide metabolite. | [storage]
Store at -20°C | [Toxicity evaluation]
Environmental exposure occurs via direct release into water or
via vaporization into the air. It is susceptible to photolysis and
is thought to have a half-life of roughly 63 days in lake water in
vitro. However, when dissolved and exposed to direct photolysis,
it has a half-life of approximately 1 day. |
|
|