| Identification | More | [Name]
Benzamide | [CAS]
55-21-0 | [Synonyms]
BENZAMIDE
BENZOIC ACID AMIDE
BENZOIC AMIDE
BENZOYLAMIDE
ai3-01031
amidkyselinybenzoove
amidkyselinybenzoove(czech)
benzenecarboxamide
Carbonamide
phenylcarboxamide
Phenylcarboxyamide
BENZAMIDE, SUBLIMED, ZONE-REFINED, 99.9%
BENZAMIDE, SUBLIMED, 99.5+%
BENZAMIDE CRYSTALLINE
BENZAMIDE INHIBITOR OF POLY (AD
BENZAMIDE 98+%
BenzamideForSynthesis
Benzamide-ring-13C6
BENZOICACIDAMINOSALT
Benzamid | [EINECS(EC#)]
200-227-7 | [Molecular Formula]
C7H7NO | [MDL Number]
MFCD00007968 | [Molecular Weight]
121.14 | [MOL File]
55-21-0.mol |
| Chemical Properties | Back Directory | [Description]
Benzamide appears as off-white crystals or powder. It is combustible and incompatible
with strong oxidising agents and strong bases. On combustion and thermal decomposition,
it emits nitrogen oxides, carbon monoxide, and carbon dioxide. | [Appearance]
Benzamide is a combustible, colorless to
beige, off-white, crystalline solid; freezing/melting
point=132 133° C. | [Melting point ]
125-128 °C (lit.) | [Boiling point ]
228°C | [bulk density]
550kg/m3 | [density ]
1.341 | [vapor pressure ]
0.000056 hPa (20 °C) | [refractive index ]
1.5323 (estimate) | [Fp ]
180°C | [storage temp. ]
Store below +30°C. | [solubility ]
ethanol: soluble50mg/mL, clear to very slightly hazy, colorless to light yellow | [form ]
Crystalline Powder | [pka]
13.0(at 25℃) | [color ]
White to almost white | [PH]
6.9 (H2O)(saturated solution) | [PH Range]
6.9 | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [Water Solubility ]
1.35 g/100 mL (20 ºC) | [Merck ]
14,1060 | [BRN ]
385876 | [LogP]
0.640 | [Uses]
Organic synthesis. | [CAS DataBase Reference]
55-21-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzamide(55-21-0) | [EPA Substance Registry System]
55-21-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed. | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
1
| [RTECS ]
CU8700000
| [Autoignition Temperature]
>500 °C | [TSCA ]
Yes | [HS Code ]
29242995 | [Hazardous Substances Data]
55-21-0(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 1125 mg/kg |
| Hazard Information | Back Directory | [General Description]
White powder. | [Reactivity Profile]
BENZAMIDE(55-21-0) reacts with azo and diazo compounds to generate toxic gases. Forms flammable gases with strong reducing agents. Mixing with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. Combustion generates toxic mixed oxides of nitrogen (NOx). | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Depresses the central nervous system;
toxic.
| [Potential Exposure]
Benzamide is used in organic
synthesis. | [Fire Hazard]
Flash point data for this chemical are not available, however BENZAMIDE is probably combustible. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including
resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical
attention. Give large quantities of water and induce
vomiting. Do not make an unconscious person vomit. | [Chemical Properties]
Benzamide is a combustible, colorless to beige, off-white, crystalline solid; freezing/melting point=132-133° C. It is slightly soluble in water, and soluble in many organic solvents.

Benzamide was used to study the mechanism of photocatalytic decomposition of aqueous solution of acetic acid, acetamide and acetonitrile in the presence of semiconductors. It was used to develop a robust screening method to study biotransformations using (+)-γ-lactamase enzyme.
| [Definition]
ChEBI: An aromatic amide that consists of benzene bearing a single carboxamido substituent. The parent of the class of benzamides. | [Preparation]
Take a mixture of 5 ml concentrated ammonia and 5 ml water in a conical flask with a well-fitting cork. Add 2 ml (2.4 g.) benzoyl chloride, cork the flask and shake vigorously. Heat generates due to the reaction, hence hold the cork securely during shaking. After 15 min not even a trace of oily benzoyl chloride remains. Filter the fine flakes, wash with cold water and recrystallise from hot water: yield, 1-5 g. Colourless crystals of benzamide.
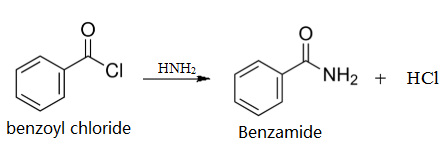
Preparation of benzamide from benzoyl chloride
| [Synthesis Reference(s)]
The Journal of Organic Chemistry, 59, p. 4114, 1994 DOI: 10.1021/jo00094a021
Chemical and Pharmaceutical Bulletin, 39, p. 1152, 1991 DOI: 10.1248/cpb.39.1152
Synthetic Communications, 20, p. 1445, 1990 DOI: 10.1080/00397919008052860 | [Biochem/physiol Actions]
Inhibits poly(ADP-ribose) polymerase (PARP). | [Clinical Use]
Benzamide on radioiodination by different labeling procedures results in large-scale production of radioiodinated benzamides having potential therapeutic application for patients with metastatic malignant melanoma. | [storage]
Color Code—Green: General storage may be used.Store in a refrigerator or a cool, dry place. | [Purification Methods]
Crystallise it from hot water (about 5mL/g), EtOH or 1,2-dichloroethane, and dry it in air. It has also been crystallised from dilute aqueous NH3, H2O, Me2CO, then *C6H6 using a Soxhlet extractor. Dry it in an oven at 110o for 8hours and store in a desiccator over 99% H2SO4. [Bates & Hobbs J Am Chem Soc 73 2151 1951, Beilstein 9 IV 725.] |
|
|