| Identification | More | [Name]
Gemfibrozil | [CAS]
25812-30-0 | [Synonyms]
2,2-DIMETHYL-5-[2,5-DIMETHYL-PHENOXY]PENTANOIC ACID
2,2-DIMETHYL-5-(2,5-XYLYLOXY) VALERIC ACID
5-(2,5-DIMETHYL-PHENOXY)-2,2-DIMETHYL-PENTANOIC ACID
5-(2,5-DIMETHYLPHYLPHENOXY)-2,2-DIMETHYLPENTANOIC ACID
GEMFIBROZIL
PENTANOIC ACID, 5-(2,5-DIMETHYLPHENOXY)-2,2-DIMETHYL-
2,2-Dimethyl-5-(2,5-Dimethylphenoxy)Valeric
2,2-dimethyl-5-(2,5-xylyloxy)-valericaci
5-(2,5-Dimethylphenoxy)-2,2-DimethylpentamoicAcid
5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoicaci
ci-719
lopid
Gemcitabine intermediates
5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic Acid, CI-719, Decrelip, Genlip, Gevilon, Lipozid, Lpur, Lopid
GEMFIBROZIL,USP
5-(2,5-DIMETHYLPHENOXY)-2,2-DIMETHYLPENTANOICACID(GEMFIBROZIL)
CI-7
Decrelip
Genlip
Lipozid | [EINECS(EC#)]
247-280-2 | [Molecular Formula]
C15H22O3 | [MDL Number]
MFCD00079335 | [Molecular Weight]
250.33 | [MOL File]
25812-30-0.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Powder | [Melting point ]
61-63°C | [Boiling point ]
158-159 C | [density ]
1.1109 (rough estimate) | [refractive index ]
1.5020 (estimate) | [storage temp. ]
2-8°C | [solubility ]
Practically insoluble in water, very soluble in methylene chloride, freely soluble in anhydrous ethanol and in methanol. | [form ]
powder | [pka]
4.75±0.45(Predicted) | [color ]
White to Off-White | [Water Solubility ]
>0.5g/L(temperature not stated) | [Usage]
A serum lipid regulating agent used as an antihyperlipoproteinemic | [Merck ]
14,4388 | [BCS Class]
2 | [CAS DataBase Reference]
25812-30-0(CAS DataBase Reference) | [IARC]
3 (Vol. 66) 1996 | [EPA Substance Registry System]
Pentanoic acid, 5-(2,5-dimethylphenoxy)-2,2-dimethyl- (25812-30-0) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R63:Possible risk of harm to the unborn child.
R62:Possible risk of impaired fertility.
R36/38:Irritating to eyes and skin .
R21:Harmful in contact with skin. | [Safety Statements ]
S36:Wear suitable protective clothing .
S53:Avoid exposure-obtain special instruction before use .
S36/37:Wear suitable protective clothing and gloves .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S25:Avoid contact with eyes . | [WGK Germany ]
3
| [RTECS ]
YV7120000
| [HazardClass ]
IRRITANT | [HS Code ]
2918992090 | [Hazardous Substances Data]
25812-30-0(Hazardous Substances Data) | [Toxicity]
LD50 in mice, rats (mg/kg): 3162, 4786 orally (Kurtz) |
| Hazard Information | Back Directory | [Description]
Gemfibrozil is a peroxisome proliferator-activated receptor α (PPARα) and PPARγ agonist (EC50s = 193.3 and 147.8 μM, respectively, in transactivation assays).1 In vivo, gemfibrozil (50 mg/kg, p.o.) reduces serum total cholesterol, triglyceride, and LDL levels in a rat model of high-cholesterol diet-induced hyperlipidemia.2 Gemfibrozil (100 mg/kg per day) reduces atherosclerotic plaque area, superoxide production, and expression of the genes encoding the NF-κB subunit p65 and chemokine (C-C) motif ligand 2 (CCL2) in ApoE-/- mice.3 Formulations containing gemfibrozil have been used in the treatment of high cholesterol. | [Originator]
Lopid,Warner Lambert,US,1982 | [Definition]
ChEBI: Gemfibrozil is an aromatic ether. It has a role as an antilipemic drug. It is functionally related to a valeric acid. | [Manufacturing Process]
With stirring, 44.1 g of isobutyric acid is added to a mixture of 51.0 g of
diisopropylamine, 23.2 g of a 57% sodium hydride dispersion in mineral oil,
and 350 ml of tetrahydrofuran. When gas evolution subsides, the mixture is
heated at reflux for 15 minutes, cooled to 0°C, and treated with 345 ml of a
1.45M solution of n-butyl lithium in heptane. After 5 hr, the mixture is
warmed one-half hour at 30°C, cooled to 0°C, and treated with 122.0 g of 3-
(2,5-xylyloxy)propyl bromide. After one more hour, it is stirred with 500 ml of
water and the aqueous phase is separated and acidified with 150 ml of 6N hydrochloric acid. The acidic mixture is extracted with ether and the ether
extract is washed with saturated sodium chloride solution, dried over
magnesium sulfate, concentrated almost to dryness, and distilled in vacuo. A
distillate of 2,2-dimethyl-5-(2,5-xylyloxy)valeric acid is collected at boiling
point 158°C to 159°C at 0.02 mm of Hg; melting point 61°C to 63°C following
crystallization from hexane.
The same product is obtained by substituting 4.4 g of lithium hydride for the
sodium hydride in the above procedure.
The same product is also obtained in the following manner. A mixture of 26.4
g of isobutyric acid, 6.0 g of magnesium oxide powder, and 250 ml of toluene
is stirred and heated at reflux with continuous removal of the water formed in
the reaction. When water formation ceases, the resulting mixture containing
magnesium isobutyrate is concentrated to one-half its original volume, cooled
in an ice bath, and treated with 31.0 g of diisopropylamine in 200 mi of dry
tetrahydrofuran and then with 179 ml of 1.68M n-butyl lithium in heptane
while the temperature is maintained below 10°C. After 15 more minutes, the
mixture is warmed at 30°C for one-half hour, cooled to 0°C to 10°C, and
treated with 75.0 g of 3-(2,5-xylyloxy)propyl bromide. The mixture is then
stirred for 18 hr at room temperature and diluted with 125 ml of 6N
hydrochloric acid and 250 ml of water. The organic phase is separated,
concentrated, and the residue distilled in vacuo to give 2,2-dimethyl-5-(2,5-
xylyloxy)valeric acid. | [Brand name]
Lopid (Pfizer);Gevilon;Hipolixan;Ipolipid;Lipur;Tenorac. | [Therapeutic Function]
Antihyperlipidemic | [World Health Organization (WHO)]
Gemfibrozil, an antihyperlipidaemic derivative of clofibrate, was
introduced in the early 1980's. It is registered in several countries for the treatment
of hyperlipidaemia unresponsive to dietary measures. (See also the WHO comment
for clofibrate). | [General Description]
Gemfibrozil, 5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acid (Lopid), is a congener of clofibratethat was used first in the treatment of hyperlipoproteinemia inthe mid-1970s. Its mechanism of action and use are similar tothose of clofibrate. Gemfibrozil reduces plasma levels ofVLDL triglycerides and stimulates clearance of VLDL fromplasma. The drug has little effect on cholesterol plasma levelsbut does cause an increase of HDL.
Gemfibrozil is absorbed quickly from the gut and excretedunchanged in the urine. The drug has a plasma half-life of1.5 hours, but reduction of plasma VLDL concentration takesbetween 2 and 5 days to become evident. The peak effect of its hypolipidemic action may take up to 4 weeks to becomemanifest. | [Biochem/physiol Actions]
Gemfibrozil selectively increases Apolipoprotein A-I levels. In yeast cells, application of gemfibrozil delays the start of DNA replication. It is used as a therapeutic agent for dyslipidemia. | [Mechanism of action]
From the chemical point of view, gemfibrozil is somewhat related to clofibrate and has
analogous pharmacological use. The primary action of gemibrozil as well as clofibrate
consists of a significant reduction in the level of very low-density proteins in the plasma
and an increase in high-density protein formation. | [Clinical Use]
Hyperlipidaemias of types IIa, IIb, III, IV and V | [Synthesis]
Gemfibrozil, 2,2-dimethyl-5-(2,5-dimethylphenoxy)valeric acid (20.2.4), is
synthesized either by hydrolysis of ethyl ester of 2,2-dimethyl-5-(2,5-dimethylphenoxy)
valeric acid (20.2.3), which is synthesized by alkylation 2,2-dimethylvaleric acid ethyl ester with 3-(2,5-dimethylphenoxy)propylbromide-1 in the presence of lithium diisopropylamide,
or by oxidation of the corresponding aldehyde (20.2.4).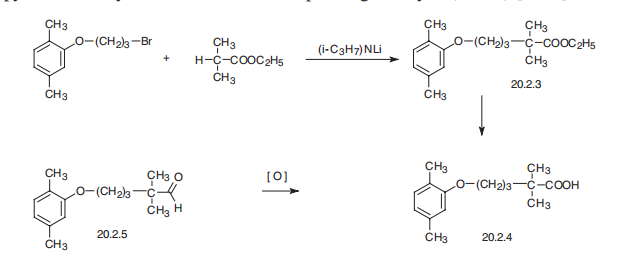
| [Veterinary Drugs and Treatments]
Gemfibrozil may be useful to reduce serum triglycerides in those
dogs or cats with hypertriglyceridemia and when diet modifications
alone have been unsuccessful. One reference (Elliott 2005)
suggests not adding drug therapy to treat hypertriglyceridemia unless
the serum triglyceride concentration exceeds 500 mg/dL with
associated clinical signs. | [Metabolism]
Gemfibrozil undergoes oxidation of a ring methyl group
to form successively a hydroxymethyl and a carboxyl
metabolite (the main metabolite). This metabolite
has a low activity compared to the mother compound
gemfibrozil and an elimination half-life of approximately
20 hours.
Gemfibrozil is eliminated mainly by metabolism.
Approximately 70% of the administered human dose is
excreted in the urine, mainly as conjugates of gemfibrozil
and its metabolites. Less than 6% of the dose is excreted
unchanged in the urine; 6% of the dose is found in faeces. |
| Questions And Answer | Back Directory | [A lipid-regulating agent]
Gemfibrozil, with the chemical name known as 2, 2-dimethyl-5-(2, 5-dimethylphenoxy) pentanoic acid, is a lipid-regulating agent belonging to clofibric acid derivatives. Appearing on the American market in 1982, it overcomes the serious side effects that the former hypolipidemic agent clofibrate has had on the liver and retains its effective role. Gemfibrozil can promote peripheral lipolysis, reduce triglyceride formation in the liver by reducing liver uptake of free fatty acids, and reduce very-low-density lipoprotein production by inhibiting the synthesis of very-low-density lipoprotein apolipoprotein. It can lower blood triglycerides and thus increases the level of blood high-density lipoprotein. It can mildly reduce the level of low-density lipoprotein cholesterol in blood. However, it may increase the level of low-density lipoprotein in type IV hyperlipoproteinemia. Controlled studies have shown that gemfibrozil can reduce the occurrences of myocardial infarction and sudden death caused by severe coronary artery disease. The agent is suitable for treatment of severe type IV or type V hyperlipoproteinemia patients with high-risk coronary heart disease who had no response to treatments such as dietary control or weight loss. It is also applicable to type II-b hyperlipoproteinemia patients with high-risk coronary heart disease who had failed to respond to treatments such as dietary control, weight loss, or other lipid-regulating medications. | [Chemical properties]
Crystallized from hexane, melting point of 61-63℃, and boiling point of 158-159℃ (2.67 Pa). Acute toxicity test: the LD50 values in mice and rats given orally were 3162 and 4786 mg/kg respectively. | [Uses]
1. Used for the treatment of hyperlipidemia. The agent is suitable for treatment of severe type IV or type V hyperlipoproteinemia patients with high-risk coronary heart disease who had no response to treatments such as dietary control or weight loss. It is also applicable to type II-b hyperlipoproteinemia patients with high-risk coronary heart disease who had failed to respond to treatments such as dietary control, weight loss, or other lipid-regulating medications.
2. Used as a lipid-regulating agent. It can significantly reduce the levels of triglyceride and cholesterol in blood, and can significantly reduce very low-density lipoprotein (VLDL) levels and increase high-density lipoprotein (HDL) levels, but it has little effect on low-density lipoprotein (LDL) levels. Clinically, it can be used in patients with all types of lipid metabolism disorders, such as primary and secondary hyperlipoproteinemias, hypercholesterolemias, hypertriglyceridemias, mixed hyperlipidemias, and lipid metabolism disorders caused by diabetes. It can also be used to prevent the occurrence of myocardial infarction.
3. The product belongs to phenoxy aromatic acid antihyperlipidemics, which is a group of drugs developed on the basis of clofibrate including fenofibrate, Etofylline Clofibrate, Bijiangzhi, gemfibrozil and so on. These drugs were similar in chemical structure. Clofibrate has rather serious adverse reactions (which include elevating the occurrence of gallstone). Improved drugs are more effective than clofibrate, with mild and fewer adverse reactions.
4. A lipid-regulating drug that can reduce cholesterol and triglyceride levels in blood. | [Synthetic route]
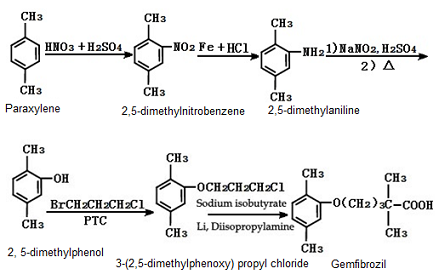
Figure 1 is a synthetic route for preparing gemfibrozil | [Production method]
(1) 1. 1-(2,5-dimethylphenoxy)-3-chloro-propane was obtained by the reaction of 2, 5-dimethylphenol with bromochloropropane. The reaction is carried out in toluene mixed with bromo-geramine in a reflux system for five hours.
2. Lithium isobutyrate was obtained by the reaction of sodium isobutyrate with lithium diisopropyl amine
3. Phenoxy acid was synthesized from 1-(2, 5-dimethylphenoxy)-3-chloro-propane and lithium isobutyrate by mixing the two intermediates slowly at 10-15℃, stirring the mixture for 15 minutes and then warming it till the temperature reaches 30℃ and keeping the reaction for five hours.
(2) Method 1: the reaction of 2, 5-dimethylphenol with 1, 3-dibromopropan was used first to obtain an intermediate 3-(2, 5-dimethylphenoxy) propyl bromide. Then 26.4 g isobutyric acid, 6.0 g magnesium oxide and 250 ml toluene were stirred into a mixture and heated under reflux, and the water formed during the heating process was continuously removed. When there was no more water forming, the solution that contains magnesium isobutyrate was concentrated to half of its original volume, and was then cooled down with an ice bath. To this solution, 31.0 g diisopropyl amine dissolved with 200 ml dry tetrahydrofuran was added first, followed by 179 ml of 1.68 mol/l n-butyllithium in pentane being added. During this period, the reaction solution was maintained at a temperature below 10℃. After 15 minutes, the solution was heated at 30℃ for 0.5 hour, then was cooled down to 0-10℃, and 75.0 g of 3-(2, 5-dimethylphenoxy) propyl bromide was added. After being stirred at room temperature for 18 hours, the solution was diluted with 125 ml of 6 mol/l hydrochloric acid and 250 ml water. Finally, the organic layer was removed and the remaining solution was concentrated and underwent vacuum distillation to give gemfibrozil with a boiling point of 158-159℃ under a vacuum of 2.67 Pa.
The product can also be obtained by the following steps: 51.0 g diisopropyl amine, 23.2 g of 57% sodium hydride in mineral oil suspension and 350 ml tetrahydrofuran were mixed and 44.1 g isobutyric acid was added into the mixture with stirring. When there was no more gas producing, the solution was heated under reflux for 15 min. Then the solution was cooled down to 0℃ and 345 ml of 1.45 mol/l n-butyllithium in pentane was added. After reacting for five hours, the solution was incubated at 30℃ for 0.5 hours, and was cooled down to 0℃ and 122.0 g 3-(2, 5-dimethylphenoxy) propyl bromide was added. After reacting for 1 hour, 500 ml water was added to the solution with stirring. Then the solution was allowed to stand to remove the aqueous layer. The remaining solution was acidified with 6mol/l hydrochloric acid and the acidic solution was extracted with ether. The extract was washed with saturated salt water, dried with anhydrous magnesium sulfate, and concentrated almost to dryness and then undergo vacuum distillation. The fraction was collect at 158-159℃ under a vacuum of 2.67 Pa, and was crystallized from hexane to give gemfibrozil of melting point 61-63℃. In the above-described method 4.4 g lithium hydride can be used to replace the sodium hydride.
Method 2: isobutyl isobutyrate was used to react with 1-chloro-3-bromopropane in the presence of lithium diisopropyl amide to produce an intermediate 5-chloro-2, 2-dimethyl-pentanoic acid isobutyl ester. The intermediate was then used to reacts with 2, 5-dimethyl phenol and in the meanwhile generated gemfibrozil by hydrolysis reaction. | [Side effects]
1. The most common adverse reactions were gastrointestinal discomfort, such as indigestion, anorexia, nausea, vomiting, a sense of fullness, and stomach discomfort; other less common adverse reactions were headache, dizziness, fatigue, skin rashes, itching, impotence, etc.
2. Occasional adverse reactions include cholelithiasis and myositis (muscle pain, or fatigue). This product belongs to clofibric acid derivatives which may cause myositis, myopathy and rhabdomyolysis, leading to elevated blood creatine-phosphokinase. The syndrome of rhabdomyolysis caused mainly showed myalgia accompanied with elevated blood creatine-phosphokinase and myoglobinuria, and in rare cases, can lead to renal failure. It may increase the risk of myopathy in patients with nephrotic syndrome or with other kidney damage that causes hypoalbuminemia, or in patients with hyperthyroidism.
3. Abnormalities in liver function tests (blood aminotransferase, lactate dehydrogenase, bilirubin, increased alkaline phosphatase) were found occasionally, which should return to normal after treatment.
4. Mild anemia and decreased white blood cell count were found occasionally, which should be stable after long term of use; occasions of severe anemia, leukopenia, thrombocytopenia, or bone marrow suppression was found quite rare. | [Contraindications]
1. Patients who are allergic to the product are contraindicated.
2. Patients with gallbladder disease or cholelithiasis are contraindicated. The agent may exacerbate the symptoms of gallbladder disease.
3. Patients with liver dysfunction or with primary biliary cirrhosis of the liver are contraindicated. The agent may promote the excretion of cholesterol and raise the level of cholesterol from high to higher.
4. Patients with severe renal insufficiency are contraindicated to use the agent as it may cause rhabdomyolysis and severe hyperkalemia.
5. Patients with decreased serum protein caused by nephrotic syndrome are contraindicated to use the agent as it may increase the risk of myopathy. | [Precautions]
1. This product may interfere with the diagnosis and cause: ① decreases in hemoglobin levels, hematocrit values and white blood cell counts, and ② increases in blood creatine-kinase, alkaline phosphatase, aminotransferase and lactate dehydrogenase.
2. During the medication the following should be checked regularly: ① blood and platelet counts, ② liver function tests, ③ lipids, and ④ blood creatine-phosphokinase.
3. Stop using the agent if the treatment is invalid after three months of therapy, or if there is any other syndrome such as cholelithiasis, significant abnormality in liver function, suspected myopathy symptoms (e.g. muscle pain, tenderness, fatigue, etc.), or significant elevated levels of blood creatine-phosphokinase being found clinically after treatment.
4. Levels of blood cholesterol and triglycerides may rebound above the original level after withdrawal of medication, and thus patients should take low-fat diet and keep monitoring the blood lipid till it returns to normal levels.
5. A variety of primary diseases that cause high blood lipid levels such as hypothyroidism and diabetes need attention and treatment during the treatment of high blood cholesterol. However, for certain medications such as estrogens, thiazide diuretics, and β-blockers which may also cause high blood lipid levels, patients do not need corresponding anti-hyperlipidemic therapy after withdrawal.
6. The agent has been classified as a level-C drug by the U.S. FDA's classifications of medications in pregnancy
7. In view of its potential carcinogenic risks, the agent should be used strictly within the specified range of indications, and should be promptly discontinued when the treatment effect is not obvious. | [Drug Interactions]
1. The product can obviously enhance the effect of oral anticoagulant drugs. When an oral anticoagulant is given in conjunction with gemfibrozil, the dosage of the oral anticoagulant should be reduced and the prothrombin time should be monitored frequently in order to adjust the dose of the anticoagulant. Its mechanism of action is unclear, which might be related to the product’s ability to replace warfarin from its binding site on the protein and enhances the role correspondingly.
2. The product, when used concomitantly with other protein-bound drugs such as furosemide, phenytoin, tolbutamide, and other sulfonylurea drugs, can also replace those drugs from the protein binding sites and thus enhance their roles. Doses of the above-mentioned drugs should be adjusted when they are taken during the lipid-lowering therapy.
3. Clofibric acid derivatives when used in combination with HMG-CoA reductase inhibitors such as lovastatin for the treatment of hyperlipidemia may increase the risks of severe muscle toxicity by both drugs, causing myopathy syndromes such as myalgia, rhabdomyolysis, and elevated blood creatine-phosphokinase, and thus should be avoided in combination.
4. Gemfibrozil, when used in combination with a bile acid-binding resin such as colestipol, should be taken at least two hours before, or after, taking the bile acid-binding resin, as the bile acid-binding resin can combine with other drugs if they are taken simultaneously and thereby affecting the absorption.
5. This product is mainly excreted by the kidneys. When it is used in combination with immunosuppressive agents such as cyclosporine, it can increase the plasma concentration of the latter as well as renal toxicity, leading to the risk of deterioration of renal function. Therefore, the immunosuppressive agent should be reduced in dose or discontinued during the therapy. Cautions should also be taken when using gemfibrozil concomitantly with other nephrotoxic drugs. |
|
|