| Identification | More | [Name]
Anisic acid | [CAS]
100-09-4 | [Synonyms]
4-ANISIC ACID
AKOS BBS-00003715
ANISIC ACID
ANISIC ACID, 4-
ANISIC ACID(P-)
FEMA 3945
'LGC' (2407)
P-ANISIC ACID
P-METHOXYBENZOIC ACID
P-METHYLHYDROXYBENZOIC ACID
RARECHEM AL BO 0059
4-methoxy-benzoicaci
Anisic acid, para
benzoicacid,4-methoxy-
Draconic acid
draconicacid
Kyselina 4-methoxybenzoova
kyselina4-methoxybenzoova
4-Methoxybenzoic Acid/p-Anisic acid
melting point standard P-anisic acid | [EINECS(EC#)]
202-818-5 | [Molecular Formula]
C8H8O3 | [MDL Number]
MFCD00002542 | [Molecular Weight]
152.15 | [MOL File]
100-09-4.mol |
| Chemical Properties | Back Directory | [Appearance]
white powder | [Melting point ]
182-185 °C(lit.)
| [Boiling point ]
275 °C
| [bulk density]
380kg/m3 | [density ]
1.385 | [vapor pressure ]
0.001Pa at 25℃ | [FEMA ]
3945 | [refractive index ]
1.571-1.576
| [Fp ]
185 °C
| [storage temp. ]
Store below +30°C. | [solubility ]
H2O: soluble2500 parts | [form ]
Powder | [pka]
4.50(at 25℃) | [color ]
White to slightly gray-beige | [Odor]
at 10.00 % in dipropylene glycol. faint putrid sweet cadaverous | [PH]
3-4 (0.3g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong oxidizing agents. | [biological source]
synthetic | [Odor Type]
animal | [Water Solubility ]
Soluble in water at 20°C 0.3g/L. Soluble in alcohol, ethyl acetate and ether. | [Usage]
Intermediates of Liquid Crystals | [JECFA Number]
883 | [Merck ]
14,666 | [BRN ]
508910 | [InChIKey]
ZEYHEAKUIGZSGI-UHFFFAOYSA-N | [LogP]
1.96 | [CAS DataBase Reference]
100-09-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Benzoic acid, 4-methoxy-(100-09-4) | [EPA Substance Registry System]
100-09-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
1
| [RTECS ]
BZ4395000
| [Autoignition Temperature]
185 °C | [Hazard Note ]
Irritant | [TSCA ]
Yes | [HS Code ]
29189090 | [Toxicity]
LD50 orally in Rabbit: > 5000 mg/kg |
| Hazard Information | Back Directory | [Description]
p-Anisic acid, also known as 4-methoxybenzoic acid ( 100-09-4) or draconic acid, is an organic acid with sweet flavor. It is one of the isomers(m-anisic acid, and o-anisic acid) of anisic acid. The term "anisic acid" often refers to this form specifically. It is a white crystalline solid which is insoluble in water, highly soluble in alcohols and soluble in ether, and ethyl acetate. P-anisic acid is produced through the oxidation of p-cresyl-methyl ether. It is ideal for use as a masking agent and fragrance ingredient in face care, body care, shower products and sun care applications.
| [Chemical Properties]
4-Methoxybenzoic acid is practically odorless. | [Chemical Properties]
white powder | [Uses]
4-Methoxybenzoic acid is used in oxidation and reduction of cytochrome c in solution through different self-assembled monolayers on gold electrodes using cyclic voltammetry. p-Anisic acid has antiseptic properties. It is also used as an intermediate in the preparation of more complex organic compounds. | [Uses]
Intermediates of Liquid Crystals | [Application]
P-anisic acid is used as raw material in many pharmaceuticals applications and has a significant role in food and cosmetics industries. It is a constituent of anise oil (Oleum anisi) that possess antiseptic, aperients, and vermifuge properties. It is effective in clearing congestion in the lungs and the respiratory tracts in conditions like asthma or bronchitis. In the synthesis of vanillin it is used as intermediate. | [Definition]
ChEBI: A methoxybenzoic acid substituted with a methoxy group at position C-4. | [Preparation]
P-anisic acid was synthesized by using n-hexyl bromide, tri(n-hexyl) amine, para-methoxy toluene with cobalt chloride hexahydrate in about 9 h and also by a catalytic oxidation processs using p-methoxy toluene and propionic acid over a catalyst comprising of CoBr2.6H2O and MnBr2.4H2O with a reaction time of 20h. After this by changing the mole ratio of cobalt and manganese, p-anisic acid was synthesized by using p-methoxy toluene with oxygen or oxygen containing gas in the presence of acetic acid. | [Synthesis Reference(s)]
Journal of Heterocyclic Chemistry, 25, p. 973, 1988 DOI: 10.1002/jhet.5570250351
Tetrahedron Letters, 34, p. 4603, 1993 DOI: 10.1016/S0040-4039(00)60635-4 | [General Description]
4-Methoxybenzoic acid is the sole source of carbon and energy for growth in the cultures of Nocardia sp. DSM 1069. | [Reactivity Profile]
4-Methoxybenzoic acid belongs to Aromatic acid hydrazides (Others include isonicotinic, benzoic, and salicylic). The reaction of thiosemicarbazide and aromatic acid hydrazides with 4H-chromen-4-imines could prepare o-hydroxyphenyl derivatives of 1H-pyrazolethioamides and 1H-pyrazol-5-ylphenols derivatives[1].
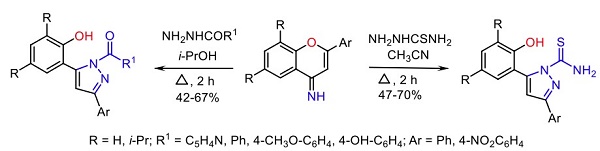
| [Hazard]
Thermal decomposition can lead to release of irritating gases and vapors, Carbon
monoxide (CO), Carbon dioxide (CO2).
| [Flammability and Explosibility]
Notclassified | [Biochem/physiol Actions]
The cytochrome P450 enzyme CYP199A4, a heme-dependent cytochrome P450 (CYP) monooxygenase enzyme, can efficiently demethylate 4-methoxybenzoic acid. Mechanistically, O-demethylation of 4-methoxybenzoic acid occurs via hydrogen abstraction by Compound I, followed by oxygen rebound to give the hydroxylated hemiacetal which spontaneously decomposes to yield 4-hydroxybenzoic acid and formaldehyde[5]. | [Safety Profile]
Poison by subcutaneous route.When heated to decomposition it emits acrid smoke andirritating vapors. | [target]
Immunology & Inflammation related | Antifection | [Solubility in organics]
Soluble in alcohol and oils.
| [Purification Methods]
Crystallise p-anisic acid from EtOH, water, EtOH/water or toluene. The S-benzylisothiuronium salt has m 189o (from EtOH). [Beilstein 10 II 91, 10 III 280, 10 IV 346.] | [References]
[1] Michael Ash (2004) Handbook of Preservatives
[2] Asim Kumar Mukhopadhyay (2004) Industrial Chemical Cresols and Downstream Derivatives
[3] https://en.wikipedia.org/wiki/P-Anisic_acid |
|
|