| Identification | More | [Name]
Diflunisal | [CAS]
22494-42-4 | [Synonyms]
2',4'-DIFLUORO-4-HYDROXY[1,1'-BIPHENYL]-3-CARBOXYLIC ACID
2',4'-DIFLUORO-4-HYDROXY-BIPHENYL-3-CARBOXYLIC ACID
5-[2,4-DIFLUOROPHENYL]SALICYLIC ACID
DIFLUSINAL
LABOTEST-BB LT00771921
[1,1'-Biphenyl]-3-carboxylic acid, 2',4'-difluoro-4-hydroxy-
1’-biphenyl)-3-carboxylicacid,2’,4’-difluoro-4-hydroxy-(
1’-biphenyl]-3-carboxylicacid,2’,4’-difluoro-4-hydroxy-[
2-(Hydroxy)-5-(2,4-difluorophenyl)benzoic acid
2-(hydroxy)-5-(2,4-difluorophenyl)benzoicacid
2',4'-Difluoro-4-hydroxy-(1',1-diphenyl)-3-carboxylic acid
2',4'-Difluoro-4-hydroxy-3-biphenylcarboxylic acid
2’,4’-difluoro-4-hydroxy-3-biphenylcarboxylicaci
2’,4’-difluoro-4-hydroxy-3-biphenylcarboxylicacid
3-Biphenylcarboxylic acid, 2',4'-difluoro-4-hydroxy-
Adomal
Difludol
Dolisal
Dolobid
Dolobil | [EINECS(EC#)]
245-034-9 | [Molecular Formula]
C13H8F2O3 | [MDL Number]
MFCD00057834 | [Molecular Weight]
250.2 | [MOL File]
22494-42-4.mol |
| Chemical Properties | Back Directory | [Melting point ]
207-209?C | [Boiling point ]
386.9±42.0 °C(Predicted) | [density ]
1.3505 (estimate) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
Practically insoluble in water, soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides. | [form ]
neat | [pka]
pKa 3.3 (H2O I=0.1) (Uncertain) | [color ]
White | [Water Solubility ]
6.186mg/L(24.99 ºC) | [CAS DataBase Reference]
22494-42-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Diflunisal(22494-42-4) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R63:Possible risk of harm to the unborn child. | [Safety Statements ]
S22:Do not breathe dust .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
DV2030000
| [Hazard Note ]
Irritant | [HS Code ]
2918290000 | [Safety Profile]
Poison by ingestion,
subcutaneous, and intraperitoneal routes.
Human systemic effects by ingestion: tolerance, and cholestatic jaundce (due to
the stoppage of the flow of bile),
agranulocytosis, increased body temperature.
An experimental teratogen. Other
experimental reproductive effects. An
analgesic and anti-inflammatory agent.
When heated to decomposition it emits
toxic fumes of F-. See also FLUORIDES. | [Toxicity]
LD50 orally in female mice: 439 mg/kg (Stone) |
| Hazard Information | Back Directory | [Description]
Diflunisal is more
potent than aspirin but produces fewer side effects and has a biological half-life three to four times greater than that
of aspirin. It is rapidly and completely absorbed on oral administration, with peak plasma levels being achieved within
2 to 3 hours of administration. It is highly bound (99%) to plasma proteins after absorption. Its elimination half-life is
8 to 12 hours, and it is excreted into urine primarily as glucuronide conjugates. The most frequently reported side
effects include disturbances of the GI system (e.g., nausea, dyspepsia, and diarrhea), dermatological reactions, and
CNS effects (e.g., dizziness and headache). | [Chemical Properties]
White Solid | [Originator]
Dolobid,Morson,UK,1978 | [Uses]
As a prostaglandin synthetase inhibitor, diflunisal exhibits analgesic, fever-reducing, and
anti-inflammatory action. It is used for long- and short-lasting symptomatic relief of low
to moderate pain in osteoarthritis and rheumatoid arthritis. | [Uses]
Salicylate; non-selective cyclo-oxygenase inhibitor; antipyretic; analgesic; anti- inflammatory. | [Definition]
ChEBI: Diflunisal is an organofluorine compound comprising salicylic acid having a 2,4-difluorophenyl group at the 5-position. It has a role as a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is an organofluorine compound and a monohydroxybenzoic acid. It is functionally related to a salicylic acid and a 1,3-difluorobenzene. | [Manufacturing Process]
A mixture of 10 g of 4-(2',4'-difluorophenyl)-phenol and 27.2 g of potassium
carbonate is exposed to carbon dioxide at 1,300 psi and 175°C. The dark
mass obtained from this carbonation is then dissolved in 300 ml of water and
200 ml of methylene chloride and the two layers separated. The water layer is
then extracted with 100 ml of methylene chloride and then acidified with 2.5
N hydrochloric acid. This mixture is then filtered and the cake dried in vacuo
to yield 5.32 g of the crude product. The crude product is then recrystallized
from benzene-methanol. An additional crystallization of this semipure material
from benzene-methanol yields analytically pure 2-hydroxy-5-(2',4'-
difluorophenyl)-benzoic acid (MP 210-211°C). | [Brand name]
Dolobid (Merck). | [Therapeutic Function]
Analgesic, Antiinflammatory | [General Description]
Diflunisal (Dolobid), is a longer acting and more potent drugthan aspirin because of its hydrophobic, 2,4-difluorophenylgroup attached to the 5-position of the salicyclic acid. In alarge-scale comparative study with aspirin, it was also bettertolerated with less GI complications than aspirin. It ismarketed in tablet form for treating mild to moderate postoperativepain as well as RA and OA.
Diflunisal is highly protein bound. Its metabolism is subjectto a dose-dependent, saturable, and capacity-limitedglucuronide formation. This unusual pharmacokineticprofile is a result of an enterohepatic circulation and the reabsorptionof 65% of the drug and its glucuronides, followedby cleavage of its unstable, acyl glucuronide back tothe active drug. Thus, diflunisal usage in patients with renalimpairment should be closely monitored. | [Mechanism of action]
Diflunisal is a weak inhibitor of both COX-1
and COX-2.
| [Pharmacology]
Peak plasma concentrations are reached
within 2 to 3 h after oral dosing. Diflunisal is
strongly bound to plasma protein (> 99 %), has
a long elimination half-life (8–12 h) and nonlinear
elimination kinetics. Hence, it is used with
an initial loading dose (1000 mg) and a lower
maintenance dose (500–1000 mg/d). | [Clinical Use]
Diflunisal (pKa 3.3) was introduced in the United States in 1982 and has gained considerable acceptance as an
analgetic and as a treatment of rheumatoid arthritis and osteoarthritis. Diflunisal is metabolized primarily to ether and
ester glucuronide conjugates. | [Side effects]
The main side effects are gastrointestinal disturbances,
headache and rash. | [Synthesis]
Diflunisal, 2??,4??-difluoro-4-hydroxy-3-byphenylcarboxylic acid (3.2.5), is
synthesized from a diazonium salt, which is synthesized from 2,4-difluoroaniline and
isoamyl nitrite, and anisole in the presence of copper (I) salts by the classic scheme of
making diaryls. The resulting 4-(2,4-difluorophenyl)anisole (3.2.3) is demethylated by
hydrogen iodide into 4-(2,4-difluorophenyl)-phenol (3.2.4). This product is reacted with
carbon dioxide in the presence of a base according to the Kolbe¨CSchmitt phenol carboxy�lation method, giving diflunisal (3.2.5) [64¨C67].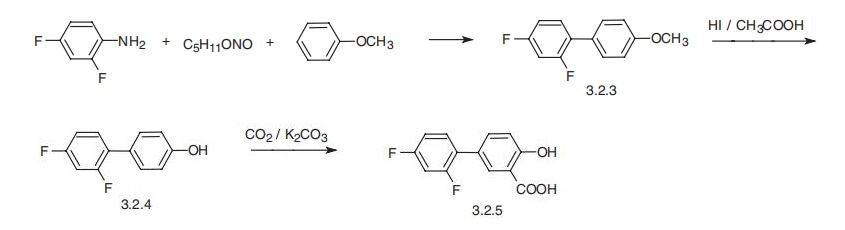
|
| Questions And Answer | Back Directory | [Outline]
Diflunisal, a nonsteroidal anti-inflammatory analgesic, is the most promising alternative to aspirin.It is used clinically for the treatment of rheumatoid arthritis, rheumatoid arthritis, osteoarthritis, sprain, strain and analgesia. Researches have indicated that diflunisal and ibuprofen are effective in the treatment of rheumatoid arthritis and degenerative arthritis. It has also been observed that diflunisal is superior to ibuprofen in improving the grip strength and relieving joint pain and tenderness of rheumatoid arthritis and degenerative arthritis in patients with rheumatoid arthritis. At the same time, diflunisal can decrease and alleviate the rheumatoid factor titre in patients with rheumatoid arthritis and stiffness,The analgesic effect of diflunisal is 7.5 to 13 times as high as aspirin.Its antipyretic effect is 1.4 times as high as aspirin, and its therapeutic effect is about 3 times as strong as aspirin. Therefore, it is suitable for treating rheumatoid arthritis, osteoarthritis, muscle sprain, strain, meniscus surgery, orthopedic and oral surgery, and primary pain caused by dysmenorrhea. It is worthy of clinical application.Diflunisal is selected from more than 500 salicylic acid derivatives by American company Merck Sharp & Do hme using the flunisal as the leading compound in 1975. It was launched in 1975. Now it is one of the Merck Co's annual sales of over 100 million US dollars. And it has been listed in more than 70 countries, such as Britain, the United States, Japan, Italy, France and other countries. It has also been recorded by the United States Pharmacopoeia and the British Pharmacopoeia. In China, the tablets and capsules of diflunisal have been approved for production.
| [Pharmacokinetics]
This product is well absorbed in oral administration, and the blood concentration 2 ~ 3H after taking will reach the peak.The half-life is proportional to the dosage, about 8 to 12h, and the binding rate of plasma protein is 90%. Oral administration of 125mg should be 3 ~ 4d and 500mg should be 7 to 9d. .The elimination of half-life of 125mg is 7 to 8h, and 500mg is 15h.Its binding rate with plasma protein in normal human body is up to 98% ~ 99%.The content of breast milk in lactating women is 2% ~ 7% of the blood concentration.It is not metabolized into salicylic acid in the body.80% to 95% of the drugs will be discharged from the urine in the form of 2 soluble glucoside complexes within 72-96h.
| [Clinical application]
This product can inhibit the synthesis of prostaglandin with analgesic, anti-inflammatory and antipyretic effects. It is used to relieve the moderate pain in bone and rheumatoid arthritis.It is also used to relieve pain and joint, muscle sprain and cancer pain after meniscus and orthopedic surgery. The drug will take effect 1h after taking and the effect lasts for 8 ~ 12h.It can also be used to treat osteoarthritis and rheumatoid arthritis etc.
| [Precaution]
- The combined use with hydrochlorothiazine, indomethacin, and paracetamol can increase the plasma concentration of these drugs.
- Long-term application can cause renal function damage and drug accumulation, so the patients with renal insufficiency should be careful in application with reduced doses.
- This drug is forbidden for patients with cardiac insufficiency, hypertension, edema, peptic ulcer and bleeding as well as for pregnant women and breast-feeding women.
- It is forbidden for those who are allergic to this product and acetyl salicylic acid.
- Used with anticoagulants, it can prolong the time of coagulation
| [Adverse reaction]
- Digestive system: anorexia, nausea, abdominal pain, abdominal distention, diarrhea, and constipation.
- Nervous system: vertigo, headache, fatigue, insomnia, lethargy, etc.
- Others: rare rash, edema, rhinitis, tinnitus, transient visual impairment, etc.
|
|
|