| Identification | More | [Name]
4-Phenylphenol | [CAS]
92-69-3 | [Synonyms]
2-DIPHENYLOL
4-BIPHENYLOL
4-HYDROXYBIPHENYL
4-HYDROXYDIPHENYL
4-PHENYLPHENOL
BIPHENYL-4-OL
PARA PHENYL PHENOL
PARAXENOL
P-HYDROXYBIPHENYL
P-HYDROXYDIPHENYL
P-PHENYLPHENOL
(1,1’-Biphenyl)-4-ol
[1,1’-biphenyl]-4-ol
[1,1'-Biphenyl]-4-ol
1-Hydroxy-4-phenylbenzene
4-Diphenylol
4-Hydroxy-1,1'-biphenyl
4-hydroxy-bipheny
l,l-biphenyl,hydroxyderivs
para-hydroxydiphenyl | [EINECS(EC#)]
202-179-2 | [Molecular Formula]
C12H10O | [MDL Number]
MFCD00002347 | [Molecular Weight]
170.21 | [MOL File]
92-69-3.mol |
| Chemical Properties | Back Directory | [Appearance]
light tan solid (odour threshold detection limit 0.7 ppm) | [Melting point ]
164-166 °C (lit.) | [Boiling point ]
321 °C (lit.) | [density ]
1.0149 (rough estimate) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.6188 (estimate) | [Fp ]
330 °F
| [storage temp. ]
0-6°C | [solubility ]
methanol: soluble50mg/mL, clear, colorless | [form ]
Flakes | [pka]
9.55(at 25℃) | [color ]
White to slightly yellow | [PH]
7 (0.7g/l, H2O, 20℃) | [Stability:]
Stable. Incompatible with strong oxidizing agents, strong bases, halogens. Combustible. | [Water Solubility ]
0.7 g/L (20 ºC) | [Merck ]
14,7305 | [BRN ]
1907452 | [LogP]
3.6 at 25℃ | [CAS DataBase Reference]
92-69-3(CAS DataBase Reference) | [NIST Chemistry Reference]
p-Hydroxybiphenyl(92-69-3) | [EPA Substance Registry System]
4-Phenylphenol (92-69-3) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,N | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R38:Irritating to the skin. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S36:Wear suitable protective clothing . | [RIDADR ]
UN3077 | [WGK Germany ]
2
| [RTECS ]
DV5850000
| [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
29071900 | [Hazardous Substances Data]
92-69-3(Hazardous Substances Data) | [Toxicity]
LD50 ipr-mus: 150 mg/kg NTIS** AD691-490 |
| Questions And Answer | Back Directory | [Chemical properties]
P-phenyl phenol appears as a white flake solid; being tasteless; mp:159 ~ 160 ℃; pure product has a mp of 166 ℃, a bp of 323 ℃, a relative density of 1.24. It is almost insoluble in water, but easily soluble in organic solvents such as alcohol, ketone and ether and alkaline solution.
| [Uses]
Biphenol, also known as p-phenyl phenol, is the intermediates of the fungicide bitertanol.It is used in the synthesis of oil-soluble resin and emulsifier; used as a component of corrosion-resistant paint, printing and dyeing carrier. P-hydroxybiphenyl synthesized red light enhancement and green light enhancement materials are one of the main raw materials for color films and are also used as analytical reagents. | [Production Methods]
Two methods for the preparation of p-hydroxybiphenyl.
Separation of byproducts of phenol production by sulfonation method
Distillation of by-products of phenol production by sulfonation, the residue contains p-phenylphenol and o-phenylphenol, the residue is first heated, distilled by vacuum, the vacuum is controlled at 53.3 ~ 66.7 kPa, the temperature gradually increases from 65 ~ 75 ℃ to more than 100 ℃, but not more than 135 ℃, and then use the different solubility of o- and para-phenylphenol in trichloroethylene for separation, that is, the mixed phenyl Phenylphenol is heated and dissolved in trichloroethylene, and the crystals of para-phenylphenol are precipitated by cooling, and the product is obtained by filtering and drying.
Biphenyl sulfonation alkali fusion method
The biphenyl is dissolved in acetic acid, sulfonated with sulfur trioxide, and the sulfonated product is separated and formed into salt with 20% NaOH aqueous solution, and then alkali melted with solid NaOH at 100-350℃, and then acidified to obtain the product. |
| Hazard Information | Back Directory | [Definition]
ChEBI: A member of the class of hydroxybiphenyls that is biphenyl carrying a hydroxy group at position 4. | [Preparation]
4-phenylphenol synthesis: Add to a 50 ml round-bottom flask, in this order, 122 mg of phenylboronic acid, 414 mg of potassium carbonate, 220 mg of 4-iodophenol, and 10 ml of deionized water. Weigh in a suitably sized container 3 mg Pd on C 10%, add 1 ml of deionized water, and stir gently by hand to form a slurry that is then transferred to the reaction flask.
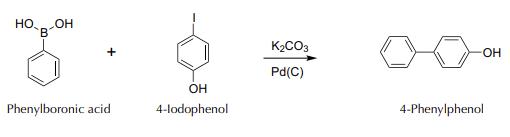
Couple the flask to a water-jacketed condenser, and reflux the mixture on a hot plate with a magnetic stirrer vigorously for 30 min (until a precipitate appears). After this time, switch off the plate and allow to cool to r.t. Add HCl 2 M to an acidic pH (check with indicator paper). Separate the resulting solid, still containing the catalyst, by filtering with a Hirsch funnel. Wash the solid with 10 ml of water. Then, in a Hirsch funnel, add 10 ml of MeOH, and collect the filtrate in a clean container. Add to the resulting MeOH solution 10 ml of deionized water to obtain the precipitate of the product. Purify by recrystallization, heating in a water bath container with the precipitate and the MeOH/H2O mixture. If necessary, add 1 to 2 ml more of hot MeOH, to finish dissolving the solid. Filter under vacuum with a Hirsch funnel, air dry the solid (can recover the next day). Weigh and calculate the yield. | [Synthesis Reference(s)]
Tetrahedron, 40, p. 4981, 1984 DOI: 10.1016/S0040-4020(01)91336-5
Tetrahedron Letters, 36, p. 125, 1995 DOI: 10.1016/0040-4039(94)02191-D | [General Description]
4-Phenylphenol undergoes enzymatic polymerization and polymer developed is characterized by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. It is the intermediate in manufacture of 4-alkyl substituted phenol-formaldehyde resins. | [Safety Profile]
Acute poison by intraperitonealroute. Questionable carcinogen with experimentalcarcinogenic and tumorigenic data. When heated todecomposition it emits acrid, irritating fumes. | [Purification Methods]
Crystallise the phenol from aqueous EtOH, *C6H6, and dry it in a vacuum over CaCl2 [Buchanan et al. J Am Chem Soc 108 7703 1986]. [Beilstein 6 IV 4600.] |
|
|