| Identification | More | [Name]
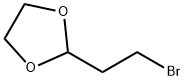
2-(2-Bromoethyl)-1,3-dioxolane | [CAS]
18742-02-4 | [Synonyms]
2-(2-BROMOETHYL)-1,3-DIOXOLANE
3-BROMOPROPIONALDEHYDE ETHYLENE ACETAL
1,3-Dioxolane, 2-(2-bromoethyl)-
4-(cyclopropylcarbonyl)-2,2-dimethylbenzeneacetonitrile
2-(2-BROMOETHYL)-1,3-DIOXOLANE, STAB.
2-(2-Bromoethyl)-1,3-dioxolane, stabilized, 96%
Bromoethyldioxolane
2-(2-Bromoethyl) 1,3-Dioxolane (Bedo)
3-bromopropanal 1,2-ethanediol acetal
1-BROMO-3,3-(ETHYLENEDIOXY)-PROPANE
2-(2-Bromoethyl)-1,3-dioxolane, 95%, stab. with silver
[2-(1,3-Dioxolane-2-yl)ethyl] bromide
2-(1,3-Dioxolane-2-yl)ethyl bromide
3-Bromopropanal ethylene acetal | [EINECS(EC#)]
242-551-1 | [Molecular Formula]
C5H9BrO2 | [MDL Number]
MFCD00003216 | [Molecular Weight]
181.03 | [MOL File]
18742-02-4.mol |
| Chemical Properties | Back Directory | [Appearance]
clear yellow to brown liquid | [Boiling point ]
68-70 °C8 mm Hg(lit.)
| [density ]
1.542 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.479(lit.)
| [Fp ]
149 °F
| [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Sparingly), Ethyl Acetate (Slightly) | [form ]
clear liquid | [color ]
brownish-yellow
| [Specific Gravity]
1.541.542 | [Water Solubility ]
immiscible | [Sensitive ]
Light Sensitive | [BRN ]
103516 | [InChIKey]
GGZQLTVZPOGLCC-UHFFFAOYSA-N | [CAS DataBase Reference]
18742-02-4(CAS DataBase Reference) | [NIST Chemistry Reference]
2-(2-Bromoethyl)-1,3-dioxolane(18742-02-4) | [Storage Precautions]
Store under inert gas |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [RIDADR ]
UN 2810 6.1/PG 3
| [WGK Germany ]
2
| [F ]
8-9 | [HazardClass ]
6.1 | [PackingGroup ]
Ⅲ | [HS Code ]
29329990 |
| Hazard Information | Back Directory | [Chemical Properties]
Clear colorless to yellow or brown liquid | [Uses]
Alkylating agent for amines,1 dithianes,2 and carboximides,3 and via the Grignard reagent, aldehydes.4 | [General Description]
Copper-catalyzed borylation of 2-(2-bromoethyl)-1,3-dioxolane with bis(pinacolato)diboron followed by treatment with potassium bifluoride affords the key organotrifluoroborate reagent. | [Purification Methods]
Dissolve it in pentane, wash with 5% aqueous NaHCO3, dry (Na2SO4), and evaporate. Distil the residue. [NMR: Büchi & Wüest J Org Chem 34 1122 1969, Kriesat & Gisvold J Pharm Sci 60 1250 1971, Beilstein 19/1 V 69.] |
|
|