| Identification | More | [Name]
Ethyl vanillin | [CAS]
121-32-4 | [Synonyms]
3-ETHOXY-4-HYDROXYBENZALDEHYDE
AKOS B004185
AKOS BBS-00003203
BOURBONAL
ETHOVAN
ETHYLPROTAL
Ethyl protocatechualdehyde 3-ethyl ether
ETHYL PROTOCATECHUIC ALDEHYDE
ETHYL VANILLIN
ETHYL VANILLIN, JAPANESE
FEMA 2464
FEMA 3107
LABOTEST-BB LT00927158
PROTOCATECHUALDEHYDE 3-ETHYL ETHER
TIMTEC-BB SBB008268
VANILLAL
3-ethoxy-
3-Ethoxy-4-hydroxybenzaldehyd
3-ethoxy-4-hydroxy-benzaldehyd
4-Hydroxy-3-ethoxybenzaldehyde | [EINECS(EC#)]
204-464-7 | [Molecular Formula]
C9H10O3 | [MDL Number]
MFCD00006944 | [Molecular Weight]
166.17 | [MOL File]
121-32-4.mol |
| Chemical Properties | Back Directory | [Appearance]
WHITE TO OFF-WHITE FINE CRYSTALLINE POWDER | [Melting point ]
74-77 °C (lit.) | [Boiling point ]
285°C | [density ]
1.1097 (rough estimate) | [vapor pressure ]
<0.01 mm Hg ( 25 °C)
| [FEMA ]
2464 | [refractive index ]
1.4500 (estimate) | [Fp ]
127°C | [storage temp. ]
Store below +30°C. | [solubility ]
2.82g/l | [form ]
Fine Crystalline Powder | [pka]
7.91±0.18(Predicted) | [color ]
White to off-white | [Odor]
at 10.00 % in dipropylene glycol. sweet creamy vanilla caramel | [biological source]
synthetic | [Odor Type]
vanilla | [Water Solubility ]
slightly soluble | [Sensitive ]
Light Sensitive | [JECFA Number]
893 | [Merck ]
14,3859 | [BRN ]
1073761 | [Stability:]
Hygroscopic | [LogP]
1.58 at 25℃ | [CAS DataBase Reference]
121-32-4(CAS DataBase Reference) | [NIST Chemistry Reference]
3-Ethoxy-4-hydroxybenzadehyde(121-32-4) | [EPA Substance Registry System]
121-32-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing . | [WGK Germany ]
1
| [RTECS ]
CU6125000
| [Hazard Note ]
Harmful/Irritant/Light Sensitive | [TSCA ]
Yes | [HS Code ]
29124200 | [Safety Profile]
Moderately toxic by
ingestion, intraperitoneal, subcutaneous, and
intravenous routes. A human skin irritant.
Mutation data reported. When heated to
decomposition it emits acrid smoke and
irritating fumes. See also ALDEHYDES and ETHERS. | [Hazardous Substances Data]
121-32-4(Hazardous Substances Data) | [Toxicity]
LD50 orally in rats: >2000 mg/kg, P. M. Jenner et al., Food Cosmet. Toxicol. 2, 327 (1964) |
| Hazard Information | Back Directory | [General Description]
Colorless crystals. More intense vanilla odor and taste than vanillin. | [Reactivity Profile]
Protect from light. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. | [Air & Water Reactions]
Slightly water soluble . | [Health Hazard]
ACUTE/CHRONIC HAZARDS: Toxic. May cause irritation on contact. | [Fire Hazard]
Combustible | [Chemical Properties]
Ethyl vanillin has an intense vanilla odor and sweet taste. The flavoring power is two to four times stronger than vanil�lin. Ethyl vanillin has been used in food since the 1930s; it enhances fruity and chocolate odor impression. Its addition is self-limiting,
as too high a level may impart an unpleasant flavor in the product; the product is not stable. In contact with iron or alkali, it exhibits
a red color and loses its flavoring power. | [Chemical Properties]
Its odor resembles that of vanillin but is
approximately three times as strong. Ethylvanillin can be prepared by method 2
as described for vanillin, using guethol instead of guaiacol as the starting material. | [Chemical Properties]
White or slightly yellowish crystals with a characteristic intense
vanilla odor and flavor. | [Chemical Properties]
WHITE TO OFF-WHITE FINE CRYSTALLINE POWDER | [Occurrence]
Not reported found in nature; it can be distinguished from vanillin because of the yellow color developed in
the presence of concentrated H2SO4. | [Uses]
Ethyl Vanillin is a flavoring agent that is a synthetic vanilla flavor
with approximately three and one-half times the flavoring power of
vanillin. it has a solubility of 1 g in 100 ml of water at 50°c. it is
used in ice cream, beverages, and baked goods. | [Uses]
In flavoring and perfumery. | [Definition]
ChEBI: A member of the class of benzaldehydes that is vanillin in which the methoxy group is replaced by an ethoxy group. | [Preparation]
From safrole by isomerization to isosafrole and subsequent oxidation to piperonal; the methylene linkage is then broken
by heating piperonal in an alcoholic solution of KOH; finally the resulting protocatechualdehyde is reacted with ethyl alcohol. From
guaethol by condensation with chloral to yield 3-ethoxy-4-hydroxyphenyl trichloromethyl carbinol; this is then boiled with an alco�holic solution of KOH or NaOH, acidified, and extracted with chloroform to yield ethyl vanillin. | [Production Methods]
Unlike vanillin, ethyl vanillin does not occur naturally. It may be
prepared synthetically by the same methods as vanillin, using
guethol instead of guaiacol as a starting material; see Vanillin. | [Aroma threshold values]
Detection: 100 ppb; recognition: 2 ppm | [Taste threshold values]
Taste characteristics at 50 ppm: sweet, creamy, vanilla, smooth and caramellic. | [Synthesis Reference(s)]
The Journal of Organic Chemistry, 44, p. 3305, 1979 DOI: 10.1021/jo01333a006 | [Flammability and Explosibility]
Nonflammable | [Pharmaceutical Applications]
Ethyl vanillin is used as an alternative to vanillin, i.e. as a flavoring
agent in foods, beverages, confectionery, and pharmaceuticals. It is
also used in perfumery.
Ethyl vanillin possesses a flavor and odor approximately three
times as intense as vanillin; hence the quantity of material necessary
to produce an equivalent vanilla flavor may be reduced, causing less
discoloration to a formulation and potential savings in material
costs. However, exceeding certain concentration limits may impart
an unpleasant, slightly bitter taste to a product due to the intensity
of the ethyl vanillin flavor. | [Safety]
Ethyl vanillin is generally regarded as an essentially nontoxic and
nonirritant material. However, cross-sensitization with other
structurally similar molecules may occur.
The WHO has allocated an acceptable daily intake for ethyl
vanillin of up to 3 mg/kg body-weight.
LD50 (guinea pig, IP): 1.14 g/kg
LD50 (mouse, IP): 0.75 g/kg
LD50 (rabbit, oral): 3 g/kg
LD50 (rabbit, SC): 2.5 g/kg
LD50 (rat, oral): 1.59 g/kg
LD50 (rat, SC): 3.5–4.0 g/kg | [storage]
Store in a well-closed container, protected from light, in a cool, dry
place. See Vanillin for further information. | [Incompatibilities]
Ethyl vanillin is unstable in contact with iron or steel, forming a redcolored, flavorless compound. In aqueous media with neomycin sulfate or succinylsulfathiazole, tablets of ethyl vanillin produced a yellow color. See Vanillin for other potential incompatibilities.
| [Regulatory Status]
GRAS listed. Included in the FDA Inactive Ingredients Database
(oral capsules, suspensions, and syrups). Included in nonparenteral
medicines licensed in the UK. |
| Questions And Answer | Back Directory | [Overview]
Vanillin (4-hydroxy-3-methoxybenzaldehyde) (121-32-4) is the primary chemical component of the extract of vanilla bean. Natural vanilla extract is a mixture of several hundred compounds in addition to vanillin. Artificial vanilla flavoring solution of pure vanillin, is usually of synthetic origin. Synthetic vanillin and ethyl vanillin are used as flavoring agents in foods, beverages, and pharmaceuticals. Ethyl vanillin (3-ethoxy-4-hydroxybenzaldehyde; EVA, Fig. 1) is more expensive and has a stronger flavor. Compared to vanillin, ethyl vanillin has an ethoxy group (-O-CH2CH3) rather than a methoxy group (-O-CH3). The largest single use of ethyl vanillin is for flavoring. It is first synthesized from eugenol found in oil of clove and afterward synthesized from lignincontaining sulfite liquor, a by-product of wood pulp processing in paper manufacture. While some ethyl vanillin is still made from lignin waste, today most synthetic vanillin is synthesized in a two-step process from the petrochemical precursors: vanillin, ethyl vanillin, and guaiacol and, glyoxylic acid. Vanilla, being the world’s most popular flavoring materials, finds extensive applications in food, beverages, perfumery and pharmaceutical industry. With the high demand and limited supply of vanilla pods and the continuing increase in their cost, numerous efforts of blending and adulteration in natural vanilla extracts have been reported.
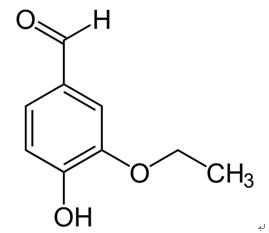
Ethyl vanillin and vanillin, the major phenolic constituents in vanilla products, are widely used as flavoring agents in foods and beverages. Ethyl vanillin, also used as a synthetic compound, is 2.5 times stronger in flavor than vanillin and used to substitute a large amount of vanillin, since it is less expensive and keeps better in storage and transport. Ethyl vanillin is converted to 3-ethoxy4-hydroxybenzaldehyde and 3-ethoxy-4-hydroxymandelic acid after dietary intake[1].
|
|
|