| Identification | More | [Name]
2,4-Dichloro-6-phenyl-1,3,5-triazine | [CAS]
1700-02-3 | [Synonyms]
1,3,5-TRIAZINE-2,4-DICHLORO, 6-PHENYL-
2-[(4-AMINO-3-CHLORO)BENZOYL]BENZOIC ACID
2,4-DICHLORO-6-PHENYL-1,3,5-TRIAZINE
4,6-DICHLORO-2-PHENYL TRIAZINE
LABOTEST-BB LT00044422
2,4-Dichloro-Phenyl-1,3,5-Triazine
2-Phenyl-4,6-dichlorotriazine
4,6-Dichloro-2-phenyl-1,3,5-triazine
6-PHENYL-1,3,5-TRIAZINE-2,4-DICHLORO | [EINECS(EC#)]
216-928-6 | [Molecular Formula]
C9H5Cl2N3 | [MDL Number]
MFCD00602464 | [Molecular Weight]
226.06 | [MOL File]
1700-02-3.mol |
| Chemical Properties | Back Directory | [Melting point ]
gt. 119.0 to 123.0 °C | [Boiling point ]
136°C/1mmHg(lit.) | [density ]
1.428±0.06 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
powder to crystal | [pka]
-1.67±0.10(Predicted) | [color ]
White to Orange to Green | [InChI]
InChI=1S/C9H5Cl2N3/c10-8-12-7(13-9(11)14-8)6-4-2-1-3-5-6/h1-5H | [InChIKey]
AMEVJOWOWQPPJQ-UHFFFAOYSA-N | [SMILES]
N1=C(C2=CC=CC=C2)N=C(Cl)N=C1Cl | [CAS DataBase Reference]
1700-02-3(CAS DataBase Reference) | [Description]
PTRZ-2Cl, namely 2,4-dichloro-6-phenyl-1,3,5-triazine, is a building block as an OLED intermediate for the synthesis of bipolar host materials such as 2,4,6-tris(4-(N,N-diphenylamino)phenyl)-1,3,5-triazine (TDPA–TRZ) for phosphorescent organic light-emitting diodes (PhOLED). | [Odor]
White to off-white powder |
| Hazard Information | Back Directory | [Chemical Properties]
White to Orange to Green powder to crystal. | [Uses]
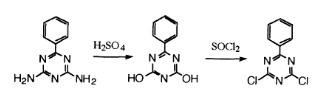
2,4-Dichloro-6-phenyl-1,3,5-triazine is a heterocyclic derivative and can be used as intermediate of synthetic materials. | [Synthesis]
2,4-Dichloro-6-phenyl-1,3,5-triazine was prepared by reaction of 36.2 g (0.21 mol) of 2,4-dihydroxy-6-phenyl-1 ,3,5-triazine with 180 g (1.51 mol) of thionyl chloride and 17.3 g of N,N-dimethylformamide (DMF) at 60°C for 3 h. The excess thionyl chloride was distilled,and the residue was poured into water to give a product.The white product was filtered off, dried, and recrys-tallized from benzene to give a yield of 22.0 g (47.4%).
 |
|
|