| Identification | More | [Name]
Benzamidine hydrochloride | [CAS]
1670-14-0 | [Synonyms]
BENZAMIDINE HCL
BENZAMIDINE HYDROCHLORIDE
BENZENECARBOXIMIDAMIDE HYDROCHLORIDE
amidinobenzenehydrochloride
benzamidiniumchloride
Benzenecarboximidamide,monohydrochloride
Benzamidine hydrochloride anhydrous
BENZAMIDINE HYDROCHLORIDE 99%
Benzamidine hydrochloride 1 M Solution
BENZAMIDINE HYDROCHLORIDE 1-HYDRATE
BENZAMIDE HCL H2O
BENZAMIDINEHYDROCHLORIDE,CRYSTAL
Benzamidine hydrochloride anhydrous 98%
benzamidine hydroxychloride
Benzamidine hydrochloride ,99%
Benzamidine·hydrochloric acid
Benzenecarboxamidine·hydrochloride
Benzamidine Hydrochloride [for Biochemical Research] | [EINECS(EC#)]
216-795-4 | [Molecular Formula]
C7H9ClN2 | [MDL Number]
MFCD00013025 | [Molecular Weight]
156.61 | [MOL File]
1670-14-0.mol |
| Chemical Properties | Back Directory | [Appearance]
white to off-white powder | [Melting point ]
86-88 °C(lit.)
| [storage temp. ]
2-8°C
| [solubility ]
DMF: 25 mg/ml
DMSO: 25 mg/ml
Ethanol: 10 mg/ml
PBS (pH 7.2): 3 mg/ml | [form ]
Powder | [color ]
White to off-white | [PH]
4.0 to 6.5(50g/L, 25 ℃) | [Water Solubility ]
soluble | [Detection Methods]
T,NMR | [BRN ]
3594959 | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions are not stable and must be prepared fresh daily | [InChIKey]
LZCZIHQBSCVGRD-UHFFFAOYSA-N | [CAS DataBase Reference]
1670-14-0(CAS DataBase Reference) | [Storage Precautions]
Moisture sensitive | [EPA Substance Registry System]
1670-14-0(EPA Substance) |
| Questions And Answer(Q&A) | Back Directory | [Preparation]
To a stainless steel rocking autoclave equipped with a stirrer is added 103.0 gm (1.0 mole) of benzonitrile, 214.0 gm (4.0 mole) of ammonium chloride, and 306.0 gm (18.0 mole) of ammonia is introduced by means of a transfer bomb. The reaction mixture is heated at 150°C for 18 hr (pressure: 1300-6500 psig), cooled, and, with appropriate precautions for the safe control of the excess ammonia, is vented to atmospheric pressure. The reaction mixture is extracted with ether to remove approximately 5% unreacted benzonitrile, and then extracted with hot acetonitrile or ethanol to separate the amidine hydrochloride from the unreacted ammonium chloride. Concentration of the latter affords 120.5 gm (77%) of benzamidine hydrochloride, m.p. 161-163°C.
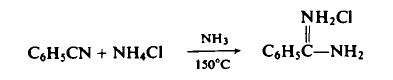
|
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection .
S36:Wear suitable protective clothing . | [WGK Germany ]
3
| [RTECS ]
CV6260000
| [F ]
3-21 | [HS Code ]
29252900 |
| Hazard Information | Back Directory | [Description]
Benzamidine hydrochloride is a reversible inhibitor of trypsin, trypsin-like enzymes, and serine proteases. A concentration of approximately 1 mM is used for general protease inhibition. To inhibit proteases from yeast, a range of 0.5 to 4.0 mM is used and it is for the most part interchangeable with pepstatin A.
In addition to being a strong competitive inhibitor of trypsin, benzamidine HCl has been also shown to be a strong competitive inhibitor of thrombin and plasmin. It was also found to be as effective as aprotinin in the prevention of glucagon degradation in human plasma. | [Chemical Properties]
white to off-white powder | [Uses]
Benzamidine is a reversible inhibitor of serine proteases, including trypsin, plasmin, and thrombin (Kis = 35, 350, and 220 μM, respectively). In addition to its use as a general serine protease inhibitor, benzamidine is used, when immobilized, to purify novel proteases. | [Biological Activity]
Benzamidine hydrochloride is an reversible competitive inhibitor of trypsin-like serine proteases, with Kis of 97 μM, 21 μM, 20 μM and 110 μM for uPA, trypsin, tryptase and factor Xa, respectively. | [Safety Profile]
Moderately toxic byintraperitoneal route. When heated to decomposition itemits toxic vapors of NOx, HCl, and Cl-. | [in vitro]
Benzamidine hydrochloride has weak inhibition for tPA and thrombin, with Kis of 750 μM and 320 μM, respectively. | [References]
1) Markwardt?et al. (1968)?Comparative studies on the inhibition of trypsin, plasmin, and thrombin by derivatives of benzylamine and benzamide; Eur. J. Biochem.,?6?502
2) Deutscher?et al.?(1990),?Maintaining protein stability; Methods Enzymol.,?182?83
3) Jaswinski?et al.?(1990),?Preparations of extracts from yeast; Methods Enzymol.,?182?154
4) Ensinck?et al.?(1972),?Use of Benzamidine as a proteolytic inhibitor in the radioimmunoassay of glucagon in plasma; J. Clin. Endocrinol. Metab.,?35?463
5) Jeffcoate?et al. (1974),?Use of benzamidine to prevent the destruction of thyrotropin-releasing hormone (TRH) by blood; Endocrinol. Metab,?38?155 |
|
|