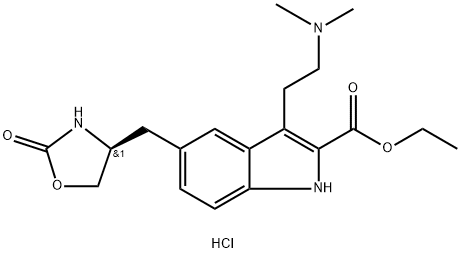
ZolMitriptan synthesis
- Product Name:ZolMitriptan
- CAS Number:868622-23-5
- Molecular formula:C19H26ClN3O4
- Molecular Weight:395.88
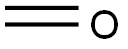
50-00-0
894 suppliers
$10.00/25g
868622-22-4
1 suppliers
inquiry

868622-23-5
8 suppliers
$165.00/10mg
Yield:-
Reaction Conditions:
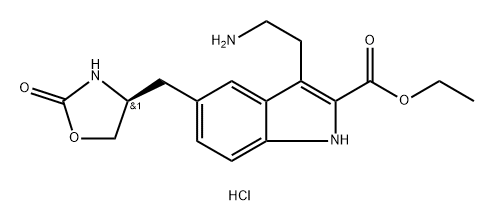
Stage #1: ethyl (S)-3-(2-aminoethyl)-5-((2-oxooxazolidin-4-yl)methyl)-1H-indole-2-carboxylate hydrochloridewith sodium cyanoborohydride;acetic acid in methanol at 10 - 15;
Stage #2: formaldehyd in methanol;water at 0;
Steps:
(S)-Ethyl-3-(2-(dimethylamino)ethyl)-5-((2-oxazolidin-4-yl)methyl)-1H-indole Carboxylate Hydrochloride 12
Concentrated hydrochloric acid (272 mL, 2.6 mol) was slowly added to a stirred solution of 4-(S)-(4-aminobenzyl)-1,3-oxazolidin-2-one (2) (200 g, 1.04 mol) in a mixture of methanol (600mL) and water (900 mL) at 5-10 °C. After stirring for 10 min, asolution of sodium nitrite (90 g, 1.3 mol) in water (60 mL) was added slowly to the reaction mixture at 5 to 0 °C, and the solution was maintained under stirring at the same temperature for 30 min. Meanwhile, in a separate vessel, sodium acetate(340 g, 4.14 mol) was added to a stirred solution of ethyl 2-acetyl-5-(1,3-dioxo-1,3-dihydro-2-isoindolyl) pentanoate (4) (3.6 g, 1.1 mol) in ethanol (3.4 L). After stirring for 1 h at room temperature (25-30 °C) the solution was cooled to 0-5 C, and the previously prepared diazotized solution was slowly added to this cooled reaction mixture and stirred for 3 h at room temperature. After the completion of reaction (monitored by TLC, using ethyl acetate as mobile phase), the reaction mass was extracted three times with dichloromethane (33 L). The combined dichloromethane layer was washed twice with water (21L) and once with brine (1 L). The organic layer was separated and concentrated. The crude residue (containing the hydrazone intermediate) was preceded for cyclization to form the indole moiety. Ethanolic HCl (1.2 L,5.04 mol) was added to this residue, and the solution was refluxed for 6 h. The reaction mass was then cooled to room temperature (25 °C) and the obtained solid was filtered and dried to furnish 7. To a stirred solution of ethyl 3-[2-(1,3-dioxo-1,3-dihydro-isoindol-2-yl)-ethyl]-5-[(4S)-2-oxo-oxazolidin-4-ylmethyl]-1H-indole-2-carboxylate (7) (200 g, 0.43 mol) in methanol (1.6 L) at room temperature (25-30 °C), hydrazine hydrate (86.8 g, 1.74 mol) was added dropwise in about 20-30 min, and the reaction mixture was maintained under stirring at 35-40 °C for 2 h. After the completion of reaction (monitored by TLC, using ethyl acetate as mobile phase), the reaction mass was cooled to 10 °C, and 2N HCl (1.9 L, 3 mol) was added to it in adropwise manner. Then the acidified reaction mixture was diluted with 1.1 L DM water, the solvent was evaporated, and the mixture was cooled to 10 °C. The solid residue was filtered off, and the filtrate was concentrated to 2.4 L volume and stirred for 15 min at 10 °C. The resulting solid was filtered and recrystallized from a mixture of methanol (3.1 L) and acetone (2.1 L) to get 8. To a stirred solution of ethyl 3-(2-aminoethyl)-5-[(4S)-2-oxo-oxazolan-4-ylmethyl]-1H-indole-2-carboxylate hydrochloride (8) (25 g, 0.07 mol) in methanol (250mL) at room temperature, 20% sodium carbonate solution was added dropwise up to pH 7. Acetic acid (10.2 g, 2.5 mol) was added to the neutralized solution glacial at the same temperature. The reaction mas swas cooled to 10-15 °C and sodium cyanoborohydride (12.65 g, 0.204 mol) was added pinch by pinch to the reaction mass under stirring at 10-15 °C. After the addition, the reaction mass was further cooled to 0 °C, and a mixture of aqueous formaldehyde solution (39%, 52.3 mL, 0.7 mol) and methanol (75 mL) was added dropwise to the stirred reaction mass. The reaction mass was stirred at the same temperature for another 60-90 min. After the completion of the reaction (via TLC, using 30% methanol and 1.5% NH3 in dichloromethane as the mobile phase), an aqueous solution of sodium carbonate [72mL of 20% (w/v)] was added at 0 °C and the reaction mass was extracted twice with dichloromethane (2400 mL). The combined organic layers were washed with DM water (2350 mL) followed by brine (450 mL) and then concentrated to 5 volumes (to a total of 125 mL) and cooled to 5-10 °C. The pH was adjusted to 5, acetone (125 ml) was added and stirred for 30 min at 5-10 °C, and the solution was filtered, washed with acetone (25 mL), and dried to get the (S)-ethyl-3-(2-(dimethyl-amino)ethyl)-5-((2-oxooxazolidin-4-yl)methyl)-1H-indole-2-carboxylate hydrochloride (12). 1H NMR (200 MHz, DMSO-d6): δ 11.70 (s, 1H),10.48 (br s, 1H), 7.85 (s, 1H), 7.69 (s, 1H), 7.40-7.36 (d, 1H, J=8.4 Hz), 7.20-7.16(d, 1H, J=8.6 Hz), 4.42-4.24 (m, 3H), 4.14-4.01 (m, 2H), 3.47-3.44 (d, 2H,J=6.2 Hz), 3.24-3.21 (d, 2H, J=7.2 Hz), 2.85 (s, 6H), 2.52-2.48 (m, 2H), 1.41-1.34 (t, 3H, J=7.2 Hz). MS: m/z 360 (M+H)+.
References:
Vujjini, Satish Kumar;Mothukuri, Vivekananda Reddy;Islam, Aminul;Bandichhor, Rakeshwar;Kagga, Mukkanti;Malakondaiah, Golla China [Synthetic Communications,2013,vol. 43,# 24,p. 3294 - 3306]