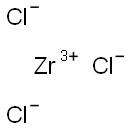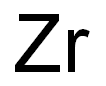
Zirconium synthesis
- Product Name:Zirconium
- CAS Number:7440-67-7
- Molecular formula:Zr
- Molecular Weight:91.22
In 2009, resources of zircon in the United States included about 14 million tons associated with titanium resources in heavy-mineral sand deposits. Phosphate and sand and gravel deposits have the potential to yield substantial amount of zircon as a future by-product. Eudialyte and gittinsite are zirconium silicate minerals that have a potential for zirconia production. Currently, identified world resources of zircon exceed 60 million tons.
The production of zirconium metal must be carried out in an atmosphere from which water vapor, oxygen, and nitrogen are rigorously excluded; otherwise, the metal becomes brittle and impossible to fabricate.
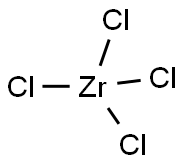
10026-11-6
225 suppliers
$24.00/50g
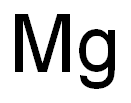
7439-95-4
0 suppliers
$11.60/0.9g
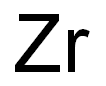
7440-67-7
0 suppliers
$36.79/10g
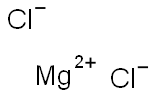
7786-30-3
573 suppliers
$20.16/250G
Yield:7440-67-7 93%
Reaction Conditions:
in meltheating at 800°C under inert gas in a closed vessel, Kroll-process; apparatus described;; separation of Mg and MgCl2 by vacuum destillation;;
References:
[Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: Zr: MVol., 23, page 90 - 92]

10026-11-6
225 suppliers
$24.00/50g
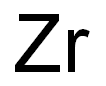
7440-67-7
0 suppliers
$36.79/10g
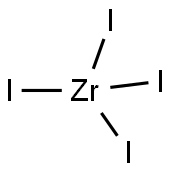
13986-26-0
77 suppliers
$56.00/1g
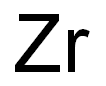
7440-67-7
0 suppliers
$36.79/10g
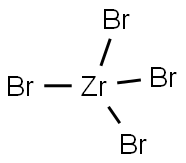
13777-25-8
65 suppliers
$76.00/5g
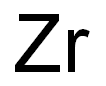
7440-67-7
0 suppliers
$36.79/10g