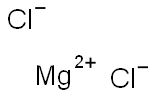
Magnesium chloride synthesis
- Product Name:Magnesium chloride
- CAS Number:7786-30-3
- Molecular formula:Cl2Mg
- Molecular Weight:95.21
Mg(OH)2(s)+2HCl→MgCl2(aq)+2H2O
It can also be prepared from MgCO3 by a similar reaction. Magnesium chloride is ionic and so will undergo electrolysis when it is molten. Electricity is carried by the movement of the ions within the melt and their discharge at the electrodes. However, solid magnesium chloride is a nonconductor of electricity because the ions are immobile.

109-69-3
445 suppliers
$20.00/100ml
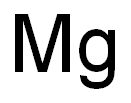
7439-95-4
0 suppliers
$16.00/0.9g
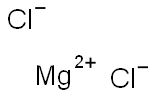
7786-30-3
573 suppliers
$20.16/250G
Yield:-
Reaction Conditions:
with iodine in octane at 130; for 2 h;Inert atmosphere;Autoclave;
Steps:
An auto-clave was packed with Mg (0.5 g, 21 mmol), few small crystalsof iodine, octane (20 ml), and 1-chlorobutane (6.5 ml, 62 mmol)and heated at130C for 2 h. The resulting -MgCl2was washedwith octane.
References:
Pirinen, Sami;Pakkanen, Tuula T. [Journal of Molecular Catalysis A: Chemical,2015,vol. 398,p. 177 - 183]

69929-18-6
2 suppliers
inquiry
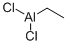
563-43-9
84 suppliers
$13.23/10ml
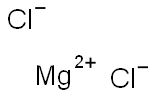
7786-30-3
573 suppliers
$20.16/250G
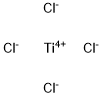
7550-45-0
336 suppliers
$10.00/10g
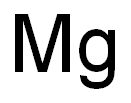
7439-95-4
0 suppliers
$16.00/0.9g
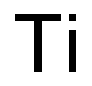
7440-32-6
230 suppliers
$20.00/1slug
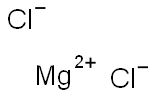
7786-30-3
573 suppliers
$20.16/250G
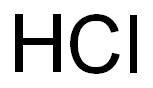
7647-01-0
0 suppliers
$10.00/10g
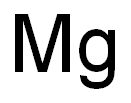
7439-95-4
0 suppliers
$16.00/0.9g
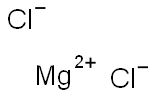
7786-30-3
573 suppliers
$20.16/250G
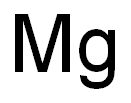
7439-95-4
0 suppliers
$16.00/0.9g
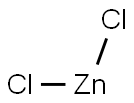
7646-85-7
578 suppliers
$10.00/25ml
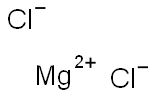
7786-30-3
573 suppliers
$20.16/250G