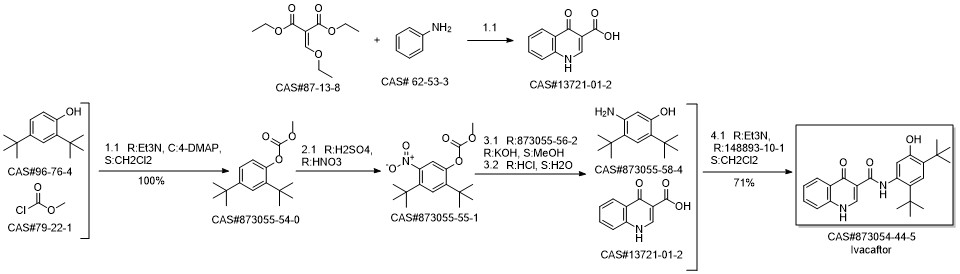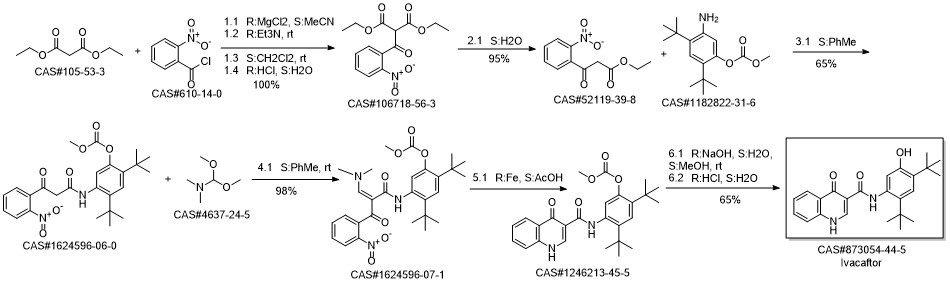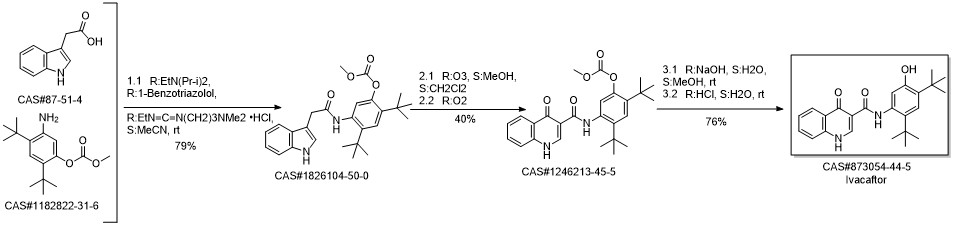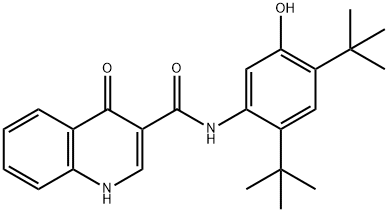
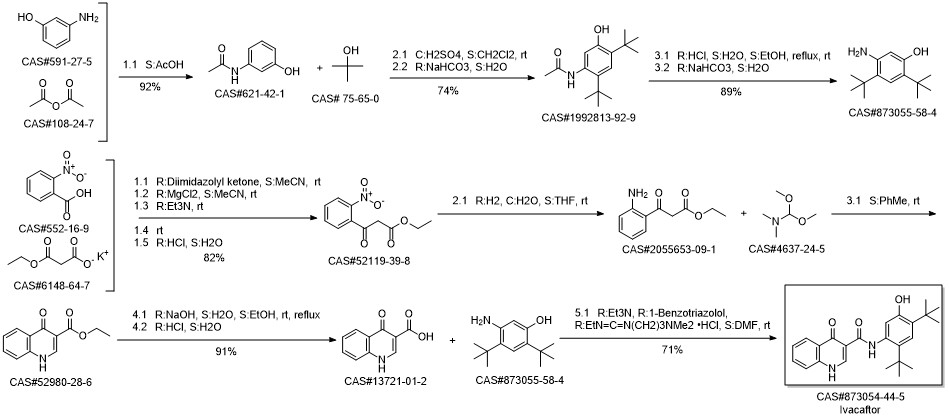
Ivacaftor synthesis
- Product Name:Ivacaftor
- CAS Number:873054-44-5
- Molecular formula:C24H28N2O3
- Molecular Weight:392.49
Reference: Zhang, Rui; Han, Guanyu; Jiang, Luobin; Shen, Yao; Yang, Rui; Mao, Yongjun; Wang, Hang. An Efficient Synthesis of Ivacaftor. Journal of Heterocyclic Chemistry. Volume 54. Issue 6. Pages 3169-3173. Journal; Online Computer File. (2017).
![Carbonic acid 5-[[(1,4-dihydro-4-oxo-3-quinolinyl)carbonyl]amino]-2,4-bis(1,1-dimethylethyl)phenyl methyl ester](/CAS/20150408/GIF/1246213-45-5.gif)
1246213-45-5
26 suppliers
inquiry

873054-44-5
285 suppliers
$28.00/1unit
Yield:873054-44-5 76%
Reaction Conditions:
with water;sodium hydroxide in methanol at 20; for 5 h;
Steps:
28 Example 28: Synthesis of ivacaftor (26):
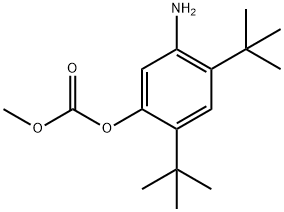
To a solution of 2,4-di-tert-butyl-5-(4-oxo-1,4-dihydroquinoline-3-carboxamido)phenyl methyl carbonate 5 (30 mg, 0.06mmol) in MeOH (2 mL) was added NaOH (5.3 mg, 0.13mmol) dissolved in H2O (2 mL), and the reaction mixture was stirred at room temperature for 5h. Reaction mass was evaporated to one third of its volume (temperature not exceeding 40°C) and acidified with aq.2N HC1 to pH 2-3. The resulting precipitate was collected by suction filtration give desired compound 7 (19 mg, 76%) as off white solid. 1H NMR (400MHz, DMSO-d6) δ = 12.88 (d, J = 6.6 Hz, 1 H), 11.81 (s, 1 H), 9.20 (s, 1 H), 8.86 (d, J = 6.6 Hz, 1 H), 8.32 (d, J = 7.8 Hz, 1 H), 7.88 - 7.65 (m, 2 H), 7.51 (t, J = 7.5 Hz, 1 H), 7.16 (s, 1 H), 7.10 (s, 1 H), 1.38 (s,9H), 1.36 (s, 9H).
References:
COUNCIL OF SCIENTIFIC & INDUSTRIAL RESEARCH;REDDY, Dumbala Srinivasa;NATARAJAN, Vasudevan;JACHAK, Gorakhnath Rajaram WO2016/181414, 2016, A1 Location in patent:Paragraph 066
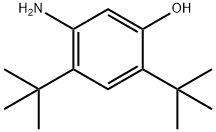
873055-58-4
153 suppliers
$40.00/100mg
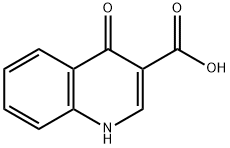
13721-01-2
256 suppliers
$11.00/250mg

873054-44-5
285 suppliers
$28.00/1unit
1182822-31-6
80 suppliers
inquiry

13721-01-2
256 suppliers
$11.00/250mg

873054-44-5
285 suppliers
$28.00/1unit

873055-58-4
153 suppliers
$40.00/100mg

873054-44-5
285 suppliers
$28.00/1unit
![3-Quinolinecarboxamide, N-[2,4-bis(1,1-dimethylethyl)-5-hydroxyphenyl]-1,4-dihydro-4-oxo-1-(phenylmethyl)-](/CAS/20210111/GIF/1622228-86-7.gif)
1622228-86-7
1 suppliers
inquiry

873054-44-5
285 suppliers
$28.00/1unit