
Voriconazole synthesis
- Product Name:Voriconazole
- CAS Number:137234-62-9
- Molecular formula:C16H14F3N5O
- Molecular Weight:349.31
Optically pure voriconazole can be prepared in a high yield by:
a) subjecting 1-(2,4-difluorophenyl)-2(1H-1,2,4-triazol-1-yl)ethanone to Reformatsky-type coupling reaction with a substituted thiopyrimidine derivative to obtain a desired (2R,3S)/(2S,3R)-enantiomeric pair;
b) removing the thiol derivative from the enantiomer to obtain racemic voriconazole; and c) isolating the racemic voriconazole by way of optical resolution using an optically active acid.
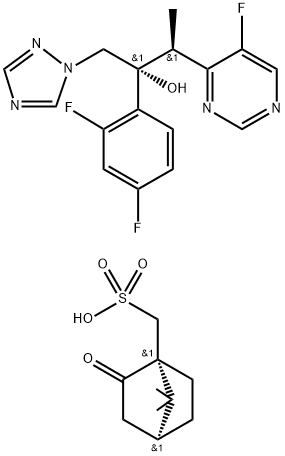
137234-71-0
52 suppliers
inquiry

137234-62-9
636 suppliers
$9.00/1mg
Yield:137234-62-9 92%
Reaction Conditions:
with sodium hydrogencarbonate in dichloromethane; for 0.5 h;
Steps:
7
Example 7. Preparation of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol (Voriconazole) [112] In 500 mL of dichloromethane, 50 g of (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidin-4-yl)-1-(1H-1,2,4-triazol-1-yl)butan-2-ol (R)-camsylateobtained from Example 6 is dissolved, and 500 mL of saturated sodium bicarbonate is introduced thereto, followed by agitation for 30 minutes. The organic layer is separated, and washed with 500 mL of saturated sodium bicarbonate and then with 500 mL of purified water. The organic layer is dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The resultant product is subjected to crystallization using 50 mL of isopropanol and 250 mL of isopropyl ether, agitated for 2 hours at 5°C, and then filtered.[113] The resultant product is dried under reduced pressure for 12 hours at 50°C to obtain 11.1 g of the title compound as a white solid (yield 92%, purity 99.95% or higher).[114] 1H-NMR (200MHz, CDCl3) δ (ppm) : 8.93(1H), 8.62(1H), 7.96(1H), 7.61-7.57(1H), 7.55(1H), 6.87-6.80(2H), 6.47(1H), 4.73(1H), 4.32(1H), 4.14(1H), 1.11(3H).
References:
WO2011/96697,2011,A2 Location in patent:Page/Page column 14-15
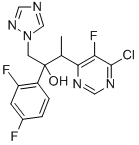
188416-35-5
187 suppliers
$145.00/1g

137234-62-9
636 suppliers
$9.00/1mg
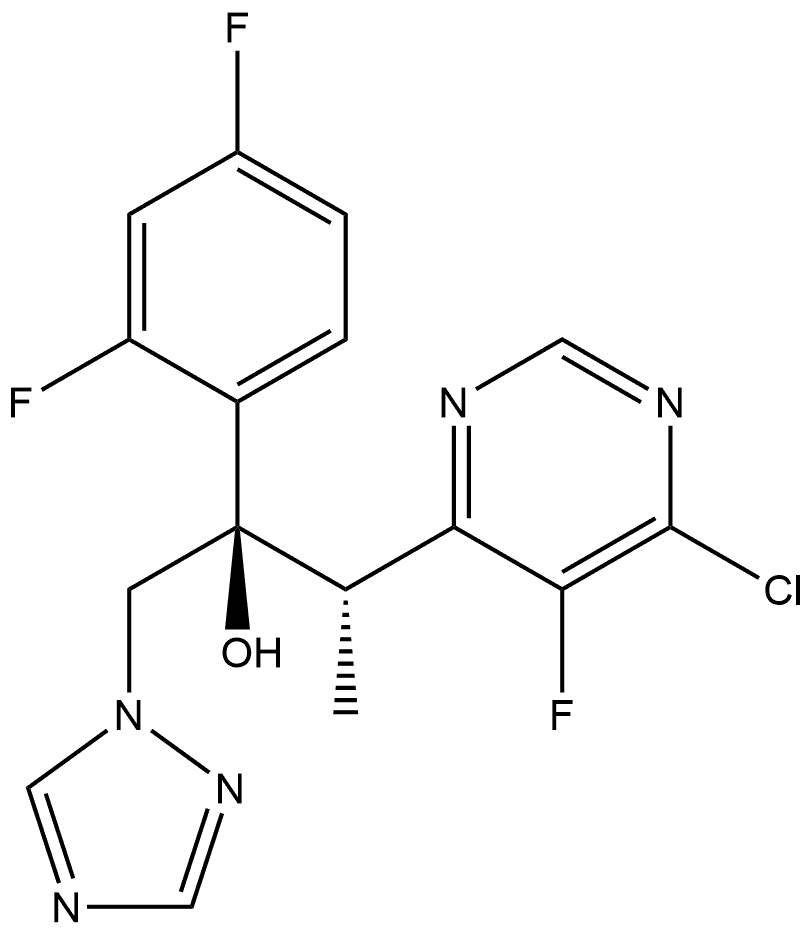
188416-38-8
0 suppliers
inquiry

137234-62-9
636 suppliers
$9.00/1mg
![Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-, (1S,4R)-, compd. with (αR,βS)-α-(2,4-difluorophenyl)-5-fluoro-β-methyl-α-(1H-1,2,4-triazol-1-ylmethyl)-4-pyrimidineethanol (1:1)](/CAS/20211123/GIF/848469-32-9.gif)
848469-32-9
1 suppliers
inquiry

137234-62-9
636 suppliers
$9.00/1mg
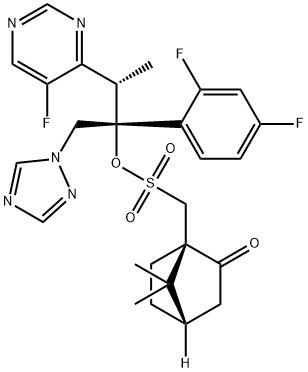
321589-01-9
1 suppliers
inquiry

137234-62-9
636 suppliers
$9.00/1mg