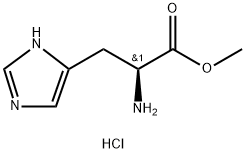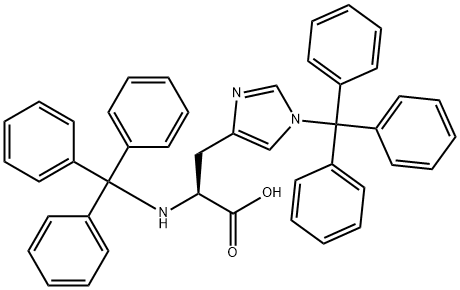
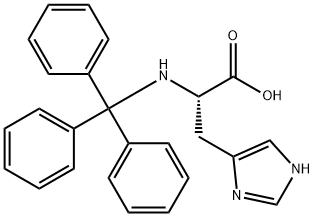
TRT-HIS(TRT)-OH synthesis
- Product Name:TRT-HIS(TRT)-OH
- CAS Number:74853-62-6
- Molecular formula:C44H37N3O2
- Molecular Weight:639.78
Yield:74853-62-6 82.5%
Reaction Conditions:
Stage #1: trityl chloride;L-histidine;zinc(II) chloride in acetonitrile at 30 - 40; for 0.166667 - 0.5 h;
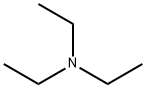
Stage #2: with triethylamine in acetonitrile;Product distribution / selectivity;
Steps:
10.A; 10.B
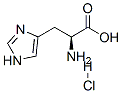
Example 10:Direct preparation of pertrityl amino acid N,Nim - ditritylhistidine (Tr-His (Tr) -OH) .Method A.; 0.20 g of histidine (1.3 mmol) are added to a solution of 1.44 g of triphenylmethyl chloride (5.2 mmol) and 0.35 g of anhydrous zinc chloride (2.6 mmol) in 15 mL of MeCN, kept under stirring at 30-350C. The solution becomes clear after 10 min. Triethylamine is added rapidly (0.72 mL, 5.2 mmol) obtaining a clear solution that, after stirring for 15 min, is poured in 200 mL of water under vigorous stirring. The pH is adjusted to 3-4 with citric acid and stirring is continued for 15 min. The suspension thus obtained is filtered and the solid is washed rapidly with water. The dissolution of this solid in acetonitrile leads to the EPO
References:
WO2007/9944,2007,A1 Location in patent:Page/Page column 27-28
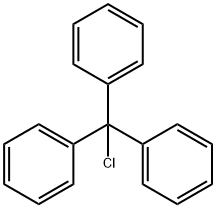
76-83-5
719 suppliers
$6.00/25g
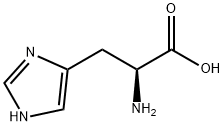
71-00-1
765 suppliers
$7.00/5g
58995-29-2
0 suppliers
inquiry

74853-62-6
18 suppliers
inquiry
645-35-2
226 suppliers
$32.00/25g

74853-62-6
18 suppliers
inquiry

76-83-5
719 suppliers
$6.00/25g
121-44-8
881 suppliers
$9.00/5g
1499-46-3
19 suppliers
inquiry

74853-62-6
18 suppliers
inquiry