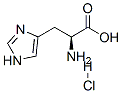
L-Histidine hydrochloride synthesis
- Product Name:L-Histidine hydrochloride
- CAS Number:645-35-2
- Molecular formula:C6H10ClN3O2
- Molecular Weight:191.62
351-50-8
363 suppliers
$10.00/1g

645-35-2
226 suppliers
$32.00/25g
Yield:645-35-2 100%
Reaction Conditions:
with hydrogenchloride;18O-labeled water in 1,4-dioxane at 100; for 48 h;Sealed tube;
Steps:
12 Example 12: Preparation of 3-((18O-L-histidyl)amino)-1-propanesulfonic acid hydrobromide (17)
Add L-histidine (1.55g, 10mmol, 1eq.)Disperse into 4M dioxane hydrochloride solution (7.5mL, 30mmol, 3eq.),Then H218O (2.0g; 18O abundance, 98%) was added.The mixture was heated in a sealed tube (oil bath, 100°C) and stirred for 24 hours,Then it was cooled to room temperature, and the solvent was removed by rotary evaporation.Disperse the obtained residue into a 4M hydrochloric acid dioxane solution (2.5mL, 10mmol, 1eq.),Then H218O (2.0g; 18O abundance, 98%) was added.Heat the mixture in a sealed tube (oil bath, 100°C) and stir for 24 hours,Then it was cooled to room temperature, and the solvent was removed by rotary evaporation.The residue was dried in vacuum,L-histidine-18O2 hydrochloride (2.32g, 100%; 18O abundance, 93.8%) was obtained. Disperse L-histidine-18O2 hydrochloride (2.32g, 10mmol, 1eq.) in methanol (50mL),Then add triethylamine (4.55g, 45mmol, 4.5eq.)And BOC anhydride (5.45g, 25mmol, 2.5eq.).The mixture was stirred at 50°C for 1 hour (the reaction system became clear),Then the reaction solution was cooled to room temperature, and the solvent was evaporated by rotary evaporation.The N,1-bis(tert-butoxycarbonyl)-L-histidine-18O2 triethylamine salt was obtained. Dissolve N,1-bis(tert-butoxycarbonyl)-L-histidine-18O2 triethylamine salt (1eq.) in dichloromethane (40mL), and then add p-nitrophenol (1.39g, 10mmol, 1eq.) and DCC (2.27g, 11mmol, 1.1eq.). After the reaction solution was stirred overnight at room temperature, the insoluble matter was removed by filtration. The filtrate was spin-dried on a rotary evaporator. The residue was purified (silica gel column chromatography; eluent, dichloromethane: ethyl acetate: petroleum ether, 2:1:7) to obtain a white solid product ester (3.0 g, 63%). The white solid (3.0 g, 6.27 mmol, 1 eq.) was dissolved in DMF (30 mL), and then sodium homotaurate (1.0 g, 6.27 mmol, 1 eq.) was added. After the mixture was stirred at room temperature overnight, the solvent was evaporated by rotary evaporation, and the residue was purified by silica gel column chromatography (eluent, methanol: dichloromethane, 1:8) to obtain a white solid 3-((N,1-bis( (Tert-Butoxycarbonyl)-18O-L-histidyl)amino)-1-propanesulfonate (2.27 g, 75.6%). The white solid was dispersed in a 1N aqueous hydrobromic acid solution (20 mL), and stirred at room temperature for 1 hour. The reaction solution was evaporated on a rotary evaporator to remove the solvent, and the obtained residue was dispersed in ethanol (30 mL). After stirring at room temperature for 1 hour, a solid was obtained by filtration. The solid was dissolved in water (5 mL), and then ethanol (30 mL) was added. After stirring at room temperature for 1 hour, a solid was obtained by filtration, and the filter cake was vacuum dried to obtain a white solid product 17 (1.5 g, 100%).
References:
CN112791078,2021,A Location in patent:Paragraph 0224-0225