
Tropicamide synthesis
- Product Name:Tropicamide
- CAS Number:1508-75-4
- Molecular formula:C17H20N2O2
- Molecular Weight:284.35
Tropicamide, N-(4-piridinylmethyl)-N-ethyl-β-hydroxy-α-phenylpropionamide (14.1.41), is synthesized by reacting O-acetyltropyl chloride with ethyl (4-piridinylmethyl)amine and the subsequent acidic hydrolysis of the acetyl group in the resulting amide (14.1.40).

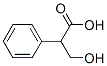
529-64-6
278 suppliers
$70.39/25 g
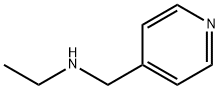
33403-97-3
250 suppliers
$10.60/1gm:

1508-75-4
325 suppliers
$5.00/100mg
Yield:1508-75-4 75.9%
Reaction Conditions:
Stage #1:Tropic acid with triethylamine;acetyl chloride in toluene at 50; for 3 h;
Stage #2: with thionyl chloride in toluene for 5 h;
Stage #3:4-(ETHYLAMINOMETHYL)PYRIDINE with α-[(acetyloxy)methyl]benzeneacetic acid;triethylamine in toluene at 0 - 10;
Steps:
5 Example 5
Preparation of Tropicamide (Compound 7):In a 250mL three-necked bottle, add 20.4g (0.123mol) of tropic acid (Compound 4), 50mL of toluene, heat to 50°C , add 0.3g (0.003mol) of triethylamine,Add 19.0g (0.24mol) acetyl chloride dropwise and react at 50°C for 3 hours,Add 20.5g (0.17mol) of thionyl chloride dropwise and continue the reaction for 5 hours,Concentrate in vacuo to a small volume, add 50mL of toluene, and cool to room temperature;In another 500mL three-necked flask, add 18.2g (0.134mol) N-ethylpyridinemethylamine pure product (Compound 1), 13.7g (0.136mol) triethylamine,100mL toluene, cooled to 0°C , add a solution of α-[(acetyloxy)methyl]benzeneacetic acid dropwise, 0-10°C reaction overnight,Add 80mL of saturated brine and wash five times. Add 27g (0.23mol) of 31% hydrochloric acid to the organic phase and heat to 90°C .After reaction overnight, liquid separation, the organic phase was washed with ammonia water, dilute hydrochloric acid, saturated brine, purified water, and the organic phase was concentrated in vacuo at 50°C The concentrated solution was recrystallized with ethyl acetate / n-heptane, the filter cake was washed with n-heptane, and dried in vacuum.26.5 g of tropicamide was obtained with a purity of 98.8% and a yield of 75.9%.
References:
Kabojinaimeisi Pharmaceutical (Shanghai) Co., Ltd.;Zhan Zujin;Wang Xilin CN111039852, 2020, A Location in patent:Paragraph 0025
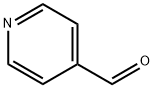
872-85-5
488 suppliers
$10.00/1g

1508-75-4
325 suppliers
$5.00/100mg
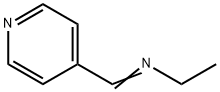
54433-74-8
1 suppliers
inquiry

1508-75-4
325 suppliers
$5.00/100mg

33403-97-3
250 suppliers
$10.60/1gm:

1508-75-4
325 suppliers
$5.00/100mg

529-64-6
278 suppliers
$70.39/25 g

1508-75-4
325 suppliers
$5.00/100mg
