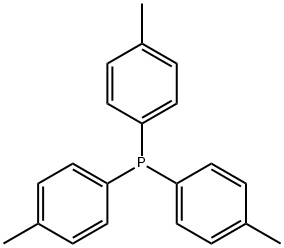
TRI-P-TOLYLPHOSPHINE synthesis
- Product Name:TRI-P-TOLYLPHOSPHINE
- CAS Number:1038-95-5
- Molecular formula:C21H21P
- Molecular Weight:304.37
2440-79-1
65 suppliers
$14.00/1g

1038-95-5
343 suppliers
$5.00/1g
Yield:1038-95-5 91%
Reaction Conditions:
with chloro-trimethyl-silane;magnesium at 20; for 4 h;
Steps:
A typical procedure for the reduction of 2a
General procedure: To a mixture of Mg powder (8 mmol, 4 mol equiv), Me3SiCl (6 mmol, 3 mol equiv), and DMI (8 mL) was added 2a (2 mmol), and the whole mixture was stirred for 2 h at room temperature. To the reaction mixture was added satd aq (NH4)2CO3, and the mixture was extracted with Et2O (10 mL 3). The combined organic layers were washed with satd aq NaCl, dried over Na2SO4, and concentrated under reduced pressure. The resultant was analyzed by 31P NMR to find that 1a and 2a were obtained in 97:3 ratio. The desired 1a was obtained in 96% yield after purification by silica gel column chromatography (hexane/AcOEt = 5:1) (Table 1, entry 1).
References:
Kuroboshi, Manabu;Kita, Toshihito;Aono, Asuka;Katagiri, Toshimasa;Kikuchi, Seiya;Yamane, Syoko;Kawakubo, Hiromu;Tanaka, Hideo [Tetrahedron Letters,2015,vol. 56,# 7,p. 918 - 920]
106-43-4
353 suppliers
$14.00/5g

1038-95-5
343 suppliers
$5.00/1g
106-38-7
413 suppliers
$14.00/5g

1038-95-5
343 suppliers
$5.00/1g
126403-71-2
0 suppliers
inquiry

1038-95-5
343 suppliers
$5.00/1g
![Silver(1+), bis[tris(4-methylphenyl)phosphine]-, nitrate (9CI)](/CAS/20211123/GIF/63692-07-9.gif)
63692-07-9
0 suppliers
inquiry

1038-95-5
343 suppliers
$5.00/1g