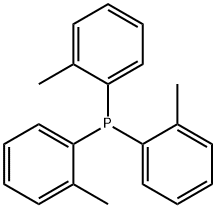
Tri(o-tolyl)phosphine synthesis
- Product Name:Tri(o-tolyl)phosphine
- CAS Number:6163-58-2
- Molecular formula:C21H21P
- Molecular Weight:304.37
Procedure: To a solution of magnesuim turnings (3.11 g, 128 mmol, 3.5 equiv) in THF (50 mL) was added a small amount of 2-bromotoluene (1 mL) and 1 iodine crystal. The reaction vessel was heated until initiation occurred, where upon a solution of the remaining 2-bromotoluene (20 g, 117 mmol, 3.2 equiv in total) in THF (100 mL) was added. The reaction was set to reflux for 2 hours (colour change to black solution), after which it was cooled to 0 oC and a solution of phosphorus trichloride (5.01 g, 36.5 mmol, 1 equiv) in THF (30 mL) was added dropwise. Upon addition completion the reaction was set to reflux for 18 hours. The reaction was allowed to cool to room temperature, whereupon NH4Cl solution was added with care. The resulting solution was extracted with ether (3 x 100 mL), dried over MgSO4, filtered and concentrated in vacuo. The resulting white solid was recrystalised from ethanol to yield Tri(o-tolyl)phosphine as a white solid. (6.8 g, 62 %)
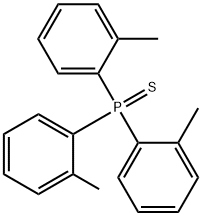
6163-61-7
0 suppliers
inquiry

6163-58-2
454 suppliers
$5.00/1g
Yield:6163-58-2 98 %
Reaction Conditions:
with sodium in toluene at 110;Inert atmosphere;Solvent;Temperature;
Steps:
4 Example 4
0.5mmol tris(o-methylphenyl)thiophosphine, 1.0mmol sodium metal,Put 1.5mL of toluene into a glass tube, raise the temperature to 110°C under the protection of nitrogen, keep the temperature for 3 hours, cool down to room temperature, wash the organic phase twice with water, remove the organic solvent under reduced pressure, and purify.Obtain 0.49mmol of tri(o-methylphenyl)phosphine,Yield 98%.
References:
CN115490727,2022,A Location in patent:Paragraph 0070-0071
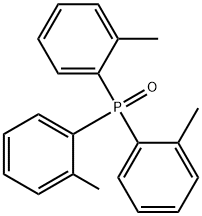
6163-63-9
30 suppliers
inquiry

6163-58-2
454 suppliers
$5.00/1g

95-46-5
304 suppliers
$10.00/10g

6163-58-2
454 suppliers
$5.00/1g
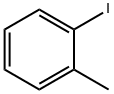
615-37-2
294 suppliers
$5.00/5g

6163-58-2
454 suppliers
$5.00/1g
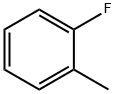
95-52-3
299 suppliers
$10.00/5g

6163-58-2
454 suppliers
$5.00/1g