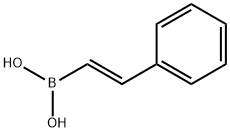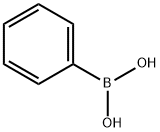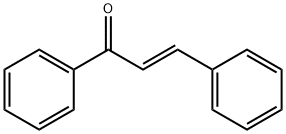
trans-Chalcone synthesis
- Product Name:trans-Chalcone
- CAS Number:614-47-1
- Molecular formula:C15H12O
- Molecular Weight:208.26
Clay-catalysed solventless synthesis of trans-chalcones
Yield:614-47-1 90%
Reaction Conditions:
with palladium diacetate;potassium carbonate;triphenylphosphine in N,N-dimethyl-formamide at 20 - 90; under 750.075 Torr; for 16 h;Inert atmosphere;Reagent/catalyst;Solvent;Concentration;
Steps:
General procedure for the synthesis of chalcones - synthesis of chalcone 3a
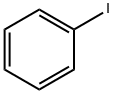
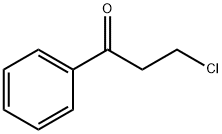
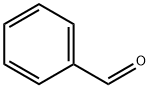
General procedure: A mixture of Pd(OAc)2 (4.5 mg, 0.02 mmol), PPh3 (11.2 mg, 0.04 mmol), iodobenzene (1a) (82 mg, 0.4mmol), 3-chloropropiophenone (2a) (87 mg, 0.5 mmol), and K2CO3 (166 mg, 1.2 mmol) in DMF (2.5 mL) was stirred under a N2 atmosphere at room temperature for 10 min, and then heated at 90 °C for 16 h. The reaction was then cooled to ambient temperature and diluted with CH2Cl2 (10 mL) before being filtered through a short pad of silica gel. The silica pad was rinsed with DCM (5 mL), and the combined filtrates were washed with brine (15 mL), dried over anhydrous Na2SO4. The solvent was then removed under reduced pressure to give the crude product as a residue, which was purified by silica gel column chromatography eluting with a mixture of petroleum ether (60-90 °C)/EtOAc (v/v = 30:1). (E)-Chalcone (3a) [21]. Yield 90%, pale yellow solid. 1HNMR (400 MHz, CDCl3): δ = 8.11 (d, J = 7.3 Hz, 2H, aromatic CH),7.90 (d, J = 15.7 Hz, 1H, CH=CHCOPh), 7.72 (dd, J = 6.3, 2.8 Hz,2H, aromatic CH), 7.69-7.55 (m, 4H, aromatic CH andCH=CHCOPh), 7.52-7.46 (m, 3H, aromatic CH).
References:
Guo, Tenglong;Jiang, Quanbin;Yu, Likun;Yu, Zhengkun [Chinese Journal of Catalysis,2015,vol. 36,# 1,p. 78 - 85]
62668-02-4
82 suppliers
$20.00/1g

614-47-1
225 suppliers
$7.00/10g