Diphenylsilane synthesis
- Product Name:Diphenylsilane
- CAS Number:775-12-2
- Molecular formula:C12H12Si
- Molecular Weight:184.31
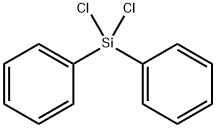
80-10-4
220 suppliers
$10.00/5g

775-12-2
214 suppliers
$10.00/1g
Yield:775-12-2 84.8%
Reaction Conditions:
with lithium aluminium tetrahydride;calcium hydride;zirconium(IV) oxide in diethylene glycol dimethyl ether at 110; for 21 h;Inert atmosphere;
Steps:
4
The 250 mL four-neck round bottom flask was dried, connected to a mechanical stirrer, a spherical condenser tube, a constant pressure funnel and a gas guiding device, and vacuum was used to replace the nitrogen gas three times, while the hot air gun was baked to remove the attached water vapor.Under a nitrogen atmosphere, 9.3 g (0.22 mol) of CaH2, 0.38 g (0.01 mol) of lithium aluminum hydride, 25 mL of diethylene glycol dimethyl ether solvent, and 40 g of ZrO 2 ceramic microspheres (φ = 0.90 mm) were added to the reaction flask.41.5 mL (0.2 mol) of Ph2SiCl2 was added to the constant pressure funnel, and slowly added dropwise to the reaction flask under mechanical stirring.After the completion of the dropwise addition, the mixture was heated to 110 ° C and reacted for 21 hours to obtain the product Ph 2 SiH 2 .According to the integral area of the 1H-NMR spectrum (Fig. 3) of the reaction system, the conversion of the product was 84.8%.
References:
Chinese Academy Of Sciences Chemical Institute;Li Yongming;Chen Yi;Xu Caihong;Wang Xinliang CN108285467, 2018, A Location in patent:Paragraph 0071-0072
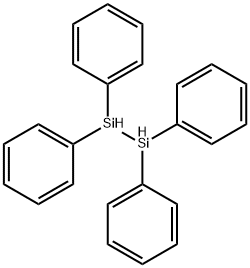
16343-18-3
45 suppliers
$60.00/50mg

775-12-2
214 suppliers
$10.00/1g
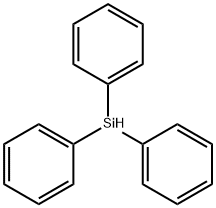
789-25-3
211 suppliers
$6.00/1g
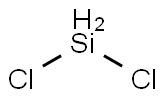
4109-96-0
57 suppliers
inquiry

775-12-2
214 suppliers
$10.00/1g
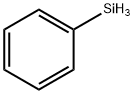
694-53-1
237 suppliers
$17.46/1G

775-12-2
214 suppliers
$10.00/1g
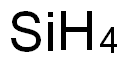
7440-21-3
227 suppliers
$30.00/100 gm

789-25-3
211 suppliers
$6.00/1g