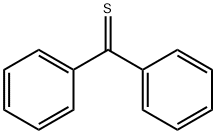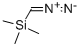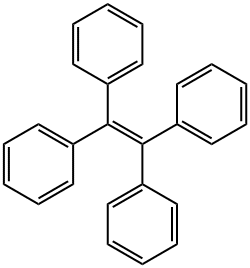
Tetraphenylethylene synthesis
- Product Name:Tetraphenylethylene
- CAS Number:632-51-9
- Molecular formula:C26H20
- Molecular Weight:332.44
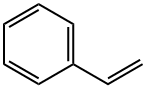
100-42-5
653 suppliers
$20.00/25mL

108-86-1
519 suppliers
$10.00/5g

632-51-9
202 suppliers
$16.00/1g
Yield:632-51-9 98%
Reaction Conditions:
with iron(III) chloride hexahydrate;P(p-CH3OC6H4)3;sodium carbonate in N,N-dimethyl-formamide at 100; for 24 h;Reagent/catalyst;
Steps:
3.1.1. Classical procedure
General procedure: Procedure A: 0.025 mmol of catalyst, 0.05 mmol of ligand, and 3.125 mmol of base were introduced into a steel tube. Next, 2.5 mmol of halogenobenzene was added together with 2.25 mmol of styrene, and then 3 mL of the solvent was nally added. The steel reactor was placed in a thermostatic oil bath maintained at 100 °C for 24 h. After the reaction, the mixture was cooled and the organic phase was extracted three times with n-hexane or cyclohexane and then with the saturated NaCl solution. The adduct obtained was then dried using Na2SO4, filtered, and placed in a 25-mL ground ask. The solvent was evaporated using a rotary evaporator(Rotavapor). The final adduct was subjected to a TLC thin-layer chromatography test using n-hexane as an eluent and treated with silica gel column chromatography with the same eluent: n-hexane. Finally, the products obtained were analyzed using the usual analysis methods.
References:
Atoui, Dhiab;Sa?d, Khemais;Ben Salem, Ridha [Turkish Journal of Chemistry,2017,vol. 41,# 4,p. 587 - 600]
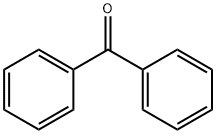
119-61-9
806 suppliers
$5.00/10g

632-51-9
202 suppliers
$16.00/1g
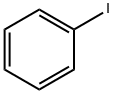
591-50-4
505 suppliers
$10.00/1g
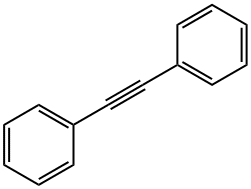
501-65-5
220 suppliers
$16.00/5g
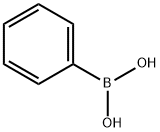
98-80-6
746 suppliers
$5.00/5g

632-51-9
202 suppliers
$16.00/1g

100-42-5
653 suppliers
$20.00/25mL

108-86-1
519 suppliers
$10.00/5g
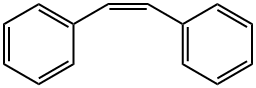
645-49-8
142 suppliers
$21.89/1g
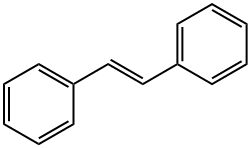
103-30-0
196 suppliers
$9.00/5g

632-51-9
202 suppliers
$16.00/1g