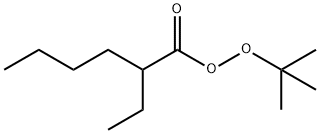
tert-Butyl peroxy-2-ethylhexanoate synthesis
- Product Name:tert-Butyl peroxy-2-ethylhexanoate
- CAS Number:3006-82-4
- Molecular formula:C12H24O3
- Molecular Weight:216.32
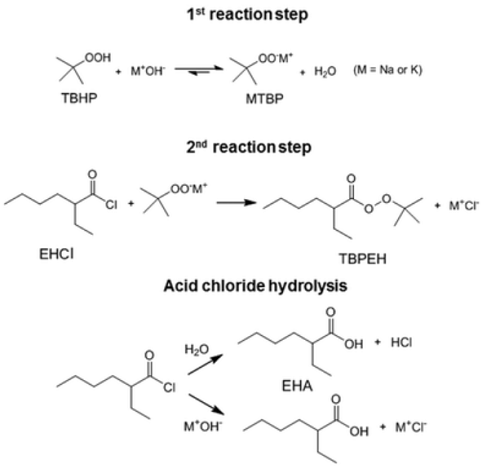
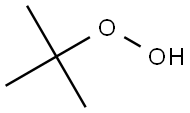
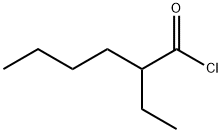
The first reaction step is the deprotonation of tert-butyl hydroperoxide (TBHP) with the base (MOH) in the aqueous phase, a homogeneous and slightly exothermic reaction. The base is usually in excess with respect to TBHP to ensure a high enough pH when the reaction has almost completed. Thanks to the TBHP acidity, the equilibrium is shifted to the right and most of the TBHP present in the aqueous phase is deprotonated. The alkali salt of TBHP (MTBP) further reacts with 2-ethylhexanoyl chloride (EHCl) to form TBPEH and a chloride salt (MCl). The reaction is biphasic with MTBP, MOH, MCl and some TBHP in the aqueous phase and EHCl, TBPEH and most of TBHP in the organic phase. The reaction between EHCl and TBHP is very slow and can be neglected[1].
Yield:3006-82-4 98.5%
Reaction Conditions:
with potassium hydroxide in water at 27 - 35;
Steps:
2
Before the start of the reaction, the loop reactor is filled with a solution of 25.8% by weight of tert-butyl hydroperoxide and 16.4% by weight of potassium hydroxide. Initially 24.6 kg/h of a solution of 70% by weight of tert-butyl hydroperoxide in water, 24.0 kg/h of a solution of 45% by weight of potassium hydroxide in water, 17.4 kg/h of water and 24.0 kg/h of 2-ethylhexanoyl chloride are then fed to the loop reactor. Cooling with cooling water keeps the internal temperature at 35° C. in the loop reactor and at 27° C. in the stirred cell reactor. The partial neutralization is effected with 5.4 kg/h of 31% by weight hydrochloric acid with addition of 6.0 kg/h of water at a temperature of 18° C. The organic phase is extracted with 26.0 kg/h of a 15% by weight aqueous solution of potassium hydroxide. This affords 29.2 kg/h of aqueous extract comprising 10.8% by weight of tert-butyl hydroperoxide and 13.4% by weight of potassium hydroxide, which are feeded into the loop reactor. From the time at which the aqueous extract is recycled into the loop reactor, the metered addition of the feedstocks is changed to 20.0 kg/h of a solution of 70% by weight of tert-butyl hydroperoxide in water, 15.4 kg/h of a solution of 45% by weight of potassium hydroxide in water and 1.4 kg/h of water. After the extraction, the organic phase is washed with 36.0 kg/h of a solution of 1% by weight of sodium sulphite and 0.2% by weight of sulphuric acid, and dried by stripping at 33° C. and 40 mbar. 31.6 kg/h of tert-butyl peroxy-2-ethylhexanoate are obtained with a purity of 99.3% (98.5% yield based on 2-ethylhexanoyl chloride).In operation with recycling of the extracted tert-butyl hydroperoxide, the molar ratio of the tert-butyl hydroperoxide to 2-ethylhexanoyl chloride feedstocks is 1.05:1
References:
US2010/22794,2010,A1 Location in patent:Page/Page column 4