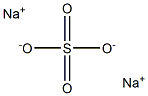
Sodium sulfate synthesis
- Product Name:Sodium sulfate
- CAS Number:7757-82-6
- Molecular formula:Na2SO4
- Molecular Weight:142.0421
Sodium sulfate is synthesized by the Mannheim process or Hargreaves process. Manheim’s process is based on Glauber’s reaction between sulfuric acid and sodium chloride: 2NaCl + H2SO4 → Na2SO4 + 2HCl↑
The process was devised by Johann Glauber to produce hydrochloric acid. Sodium sulfate is isolated from the solution by fractional crystallization.
Hargreaves’ process also was developed to produce hydrochloric acid. It is a variation of Mannheim’s method. In this method, sulfur dioxide is used instead of sulfuric acid. The reaction is as follows: 4NaCl + 2SO2 + O2 + 2H2O → 2Na2SO4 + 4HCl↑
Sodium sulfate also is obtained as a byproduct of manufacturing phenol by caustic fusion.
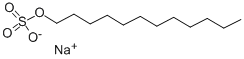
151-21-3
1040 suppliers
$6.00/25g

7757-82-6
825 suppliers
$6.00/5g
Yield:-
Reaction Conditions:
with AgNO3 or NaNO3;SnCl2*2H2O;cetyltrimethylammonium bromide in N,N-dimethyl-formamide;refluxing a mixt. of dodecylsodium sulfate, nitrate, SnCl2*2H2O, cetyltrimethylammonium bromide in DMF at 160°C for 60 min, cooling to room temp.; centrifugation, washing with 2-propanol;
References:
Pu, Ying-Chih;Hwu, Jih Ru;Su, Wu-Chou;Shieh, Dar-Bin;Tzeng, Yonhua;Yeh, Chen-Sheng [Journal of the American Chemical Society,2006,vol. 128,# 35,p. 11606 - 11611]
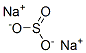
7757-83-7
736 suppliers
$13.50/250G

7757-82-6
825 suppliers
$6.00/5g
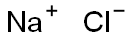
7647-14-5
1178 suppliers
$5.00/500g
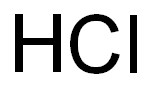
7647-01-0
0 suppliers
$10.00/10g

7757-82-6
825 suppliers
$6.00/5g
7446-09-5
139 suppliers
$195.00/25ML
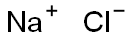
7647-14-5
1178 suppliers
$5.00/500g

7757-82-6
825 suppliers
$6.00/5g