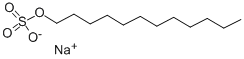
Sodium dodecyl sulfate synthesis
- Product Name:Sodium dodecyl sulfate
- CAS Number:151-21-3
- Molecular formula:C12H26O4S.Na
- Molecular Weight:289.39
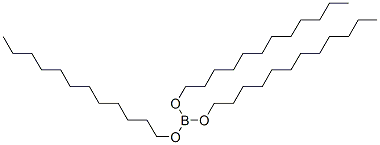
2467-15-4
8 suppliers
inquiry

151-21-3
1041 suppliers
$6.00/25g
Yield:151-21-3 451 g
Reaction Conditions:
Stage #1: tris(dodecyloxy)boranewith sulfuric acid in 1,2-dichloro-ethane;Cooling with ice;
Stage #2: with sodium hydroxide in methanol;1,2-dichloro-ethane; for 0.5 h;Cooling with ice;
Steps:
3 Example 3
The solid boron ester dissolves in 300mL dichloroethane and a homogenous mixture is acquired. While tridodecyl borate in the reactor transferred into an ice-bathe is being stirred with a mechanical mixer, 150 g H2SO4(98%) is added dropwise. Precipitated boric acid is filtered through a sintered glass funnel and thefiltered liquid is transferred to 2L volume Erlenemeyer flask cooled in ice-bath. Concentrated methanol solution comprising60g NaOH solution previously prepared is added dropwise for 30 minutes on boron ester stirred vigorously. Precipitation of sodium dodecyl sulfate is observed clearly during this addition. When the product is filtered, milky white obtainedsolids are washed with cold methanol solution and dried for 3 hours at 50°C under vacuum. 451 g product is yielded quantitatively. H-NMR spectrum of the product re-crystallized from ethanol represents exactly the spectrum of the sodiumdodecyl sulfate (SDS) in the literature.
References:
EP2851362,2015,A1 Location in patent:Paragraph 0036

112-53-8
462 suppliers
$10.00/5g

151-21-3
1041 suppliers
$6.00/25g
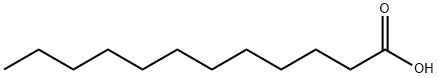
143-07-7
766 suppliers
$5.00/25g

151-21-3
1041 suppliers
$6.00/25g
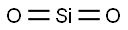
7631-86-9
674 suppliers
$14.30/50g

151-21-3
1041 suppliers
$6.00/25g
557-04-0
925 suppliers
$5.00/25g