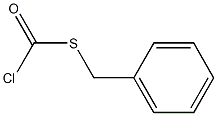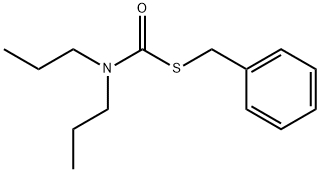
PROSULFOCARB synthesis
- Product Name:PROSULFOCARB
- CAS Number:52888-80-9
- Molecular formula:C14H21NOS
- Molecular Weight:251.39
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;nitrogen in water;toluene
Steps:
2 Preparation of N,N-dipropyl-S-benzylthiocarbamate
Preparation of N,N-dipropyl-S-benzylthiocarbamate A 250 ml flask was fitted with a magnetic stirrer, a thermometer, a gas inlet tube, and a pressure-equalizing addition funnel. The gas inlet tube was connected with a "T" to a nitrogen source and a caustic scrubber. The flask was placed under a nitrogen atmosphere and was charged with 60 ml of a 10% sodium hydroxide solution, 50 ml of toluene, and 15 ml of dipropylamine. Benzyl chlorothioformate (20 g) was added dropwise over a 5-minute period with cooling in an ice-water bath, and stirring was continued for an additional 30 minutes. The reaction mixture was washed once with 60 ml of warm water and twice with 60 ml of 3N hydrochloric acid solution. The organic phase was concentrated under reduced pressure to give 26.0 g of technical N,N-dipropyl-S-benzylthiocarbamate (98.65 of theory) which was 97.2 weight percent N,N-dipropyl-S-benzylthiocarbamate, 0.1 area percent benzyl chloride, 0.4% dipropylamine, 0.5% benzyl sulfide, 0.3% benzyl disulfide and 0.1 benzyl dithiocarbonate.
References:
Stauffer Chemical Company US4740623, 1988, A
201230-82-2
1 suppliers
inquiry
100-44-7
660 suppliers
$13.50/250G
142-84-7
254 suppliers
$10.00/5mL

52888-80-9
69 suppliers
$45.00/1mg

124-38-9
129 suppliers
$175.00/23402

142-84-7
254 suppliers
$10.00/5mL
100-53-8
253 suppliers
$10.00/1g

52888-80-9
69 suppliers
$45.00/1mg
201230-82-2
1 suppliers
inquiry
100-39-0
439 suppliers
$10.00/10 g

142-84-7
254 suppliers
$10.00/5mL

52888-80-9
69 suppliers
$45.00/1mg