
Potassium synthesis
- Product Name:Potassium
- CAS Number:7440-09-7
- Molecular formula:K
- Molecular Weight:39.1
Although the left-hand side of the equation is favored thermodynamically, the escape of the potassium vapors causes the reaction to proceed very efficiently to the right. The potassium vapors are condensed and the product normally contains sodium metal as the only major impurity up to about 1 % by weight. This product is sometimes purified by fractionating it in a 38 ft high 316 stainless steel tower equipped with a reflux return reservoir. The condensate is potassium metal of 99.99 % purity.
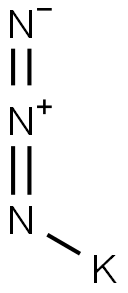
20762-60-1
52 suppliers
$59.70/25g

7440-09-7
0 suppliers
$59.10/1g
Yield:7440-09-7 80%
Reaction Conditions:
in neat (no solvent)byproducts: N; heating 10-12g KN3 (fine powder) to 355°C (high vacuum, electrically heated tube from instrument glass (Jena)), decompn. starts sometimes 3-4h after obtaining temp., synthesis lasts some d;; spectroscopically pure and gas-free K obtained;;
References:
Suhrmann, R.;Clusius, K. [Zeitschrift fur anorganische Chemie,1926,vol. 152,p. 52 - 52] [Gmelin Handbuch der Anorganischen Chemie,Gmelin Handbook: K: MVol.1, 9, page 63 - 65]
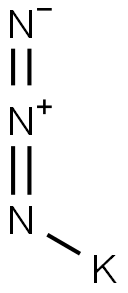
20762-60-1
52 suppliers
$59.70/25g

7727-37-9
117 suppliers
$268.00/56l

7440-09-7
0 suppliers
$59.10/1g
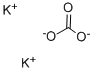
584-08-7
1230 suppliers
$5.00/100g

7440-09-7
0 suppliers
$59.10/1g
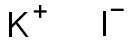
7681-11-0
1257 suppliers
$10.00/5g

7440-09-7
0 suppliers
$59.10/1g