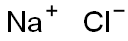
Sodium synthesis
2synthesis methods
- Product Name:Sodium
- CAS Number:7440-23-5
- Molecular formula:Na
- Molecular Weight:22.99
Sodium metal is produced commercially today by the electrolysis of fused sodium
chloride in a Downs cell. The Castner cell was the first commercially successful cell and
remained the principal source of sodium metal from about 1891 until being superseded by
the Downs cell6. This latter cell, introduced at duPont's Niagara Falls plant about 1921,
consists of a steel, refractory-brick-lined vessel with a graphite anode projecting upward from the bottom and a cast steel cathode surrounding the anode with an electrode spacing
of about 4 cm. The electrolyte is a eutectic mixture of roughly 40 % sodium chloride and
60 % calcium chloride, chosen so that the melting point of the mixed salt system is about
580°C, the operating temperature of the cell. The voltage drop across the cell is about
7 V and the current efficiency is roughly 85 %. As current flows through the molten salt
mixture, chlorine is liberated at the anode, and both sodium metal and calcium metal are
formed at the cathode; an iron gauze diaphragm between the electrodes prevents recombination.
The chlorine vapors from the anode flow overhead to a nickel collector dome
under slight vacuum, whence it is led to the chlorine purification and collection system.
The cathode product, a solution of calcium metal in liquid sodium, floats on the molten
salt bath and ascends through a vertical riser pipe into a collector vessel. As the solution
rises in the pipe, it is cooled to a temperature where calcium metal crystallizes, falls back into
the cell, and there reacts with the electrolyte. The sodium metal is filtered at 105-110°C to
remove calcium metal as well as small amounts of oxides and chlorides.
Many other methods for producing sodium metal have been devised, including electrolysis of sodium carbonate, borate, nitrate, etc., but only the Castner-type cell based on electrolysis of sodium hydroxide has had any appreciable commercial success. Today, only the Downs-type cell is used commercially to produce sodium metal in most countries; a few Castner-type cells are still operating, but total production is quite small.
Many other methods for producing sodium metal have been devised, including electrolysis of sodium carbonate, borate, nitrate, etc., but only the Castner-type cell based on electrolysis of sodium hydroxide has had any appreciable commercial success. Today, only the Downs-type cell is used commercially to produce sodium metal in most countries; a few Castner-type cells are still operating, but total production is quite small.
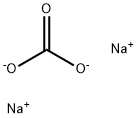
497-19-8
1429 suppliers
$13.00/300G
7440-23-5
0 suppliers
$34.60/25g