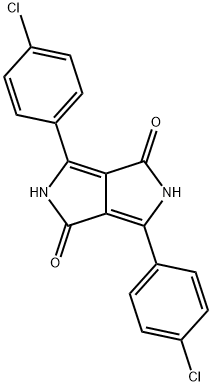
Pigment Red 254 synthesis
- Product Name:Pigment Red 254
- CAS Number:84632-65-5
- Molecular formula:C18H10Cl2N2O2
- Molecular Weight:357.19
623-03-0
475 suppliers
$6.00/25g
123-25-1
473 suppliers
$5.00/25g

84632-65-5
186 suppliers
$50.00/1g
Yield:84632-65-5 81%
Reaction Conditions:
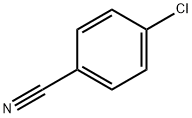
Stage #1:4-Cyanochlorobenzene with sodium tert-pentoxide in tert-Amyl alcohol at 90; for 0.0833333 h;
Stage #2: with 1-butyl-3-methylimidazolium Tetrafluoroborate in tert-Amyl alcohol for 0.166667 h;
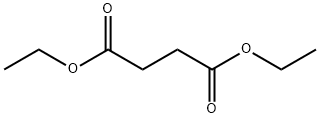
Stage #3:succinic acid diethyl ester in tert-Amyl alcohol at 30; for 14 h;Reagent/catalyst;Temperature;Solvent;
Steps:
(1b) Preparation of 3,6-Bis(4-chlorophenyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione
General procedure: Para-chlorobenzonitrile (2.76 g, 20 mmol) was added to sodium t-amyl oxide (from sodium, 0.40 g, 17.4 mmol) indry t-amyl alcohol (5ml), stirred for 5 min at 90°C, and ionic liquid (0.1 ml) was added to the reaction mixture and stirred for 10 min. Diethyl succinate (2 ml, 10 mmol) was added slowly at 30°C. Reaction was monitored by TLC and thes olution was neutralized with HCl. The precipitated pigment was diluted using 20 ml of water and filtrated. Filter cake was rinsed by methanol to remove the remained ionic liquids and dried at 80°C in the vacuum.1b: Red powder; m.p. > 300°C; IR (KBr) (max cm-1):3436 (NH); 2931(CH); 1635 (C=O); 1392, 1169(C-N);max/nm (DMSO) 520, 482; 1H NMR (500 MHz, DMSO-d6):H 7.65 (4H, d, JHH 13.3 Hz, 4CH), 7.87 (4H, d, JHH 13.3 Hz,4 CH).
References:
Nourmohammadian, Farahnaz;Shamekhi, S. Shiva [Letters in Organic Chemistry,2013,vol. 10,# 2,p. 131 - 138]

623-03-0
475 suppliers
$6.00/25g
924-88-9
192 suppliers
$7.00/10g

84632-65-5
186 suppliers
$50.00/1g

623-03-0
475 suppliers
$6.00/25g

924-88-9
192 suppliers
$7.00/10g
100-47-0
375 suppliers
$14.14/25gm:
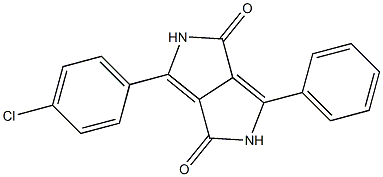
88949-37-5
3 suppliers
inquiry

84632-65-5
186 suppliers
$50.00/1g
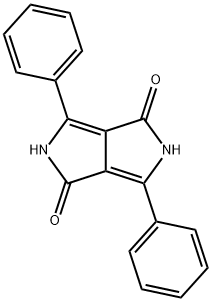
54660-00-3
52 suppliers
$45.00/50mg