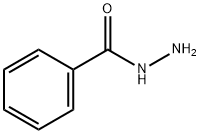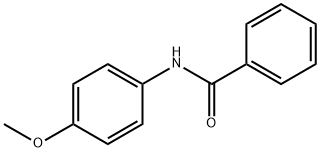
P-BENZANISIDIDE synthesis
- Product Name:P-BENZANISIDIDE
- CAS Number:7472-54-0
- Molecular formula:C14H13NO2
- Molecular Weight:227.26
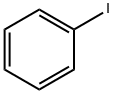
591-50-4
491 suppliers
$10.00/1g
201230-82-2
1 suppliers
inquiry

104-94-9
490 suppliers
$9.00/1g

7472-54-0
62 suppliers
$12.00/1mg
Yield:7472-54-0 100%
Reaction Conditions:
with triethylamine in N,N-dimethyl-formamide at 100; under 750.075 Torr; for 8 h;Schlenk technique;Inert atmosphere;
Steps:
2.3.2. Catalytic reactions at atmospheric pressure
General procedure: In a typical experiment a solution containing the palladiumcatalyst (with 6 mol Pd-content) was placed in a Schlenk-tube.Under argon, 0.2 mmol (22.5 l) iodobenzene (1), 0.5 mmol aminereagent, 0.7 mmol (100 l) triethylamine and 1 ml solvent wasadded and the atmosphere was changed to carbon monoxide. Thereaction mixture was heated with stirring in an oil bath at 100Cand was analysed by gas chromatography.
References:
Urbán, Béla;Papp, Máté;Srankó, Dávid;Skoda-F?ldes, Rita [Journal of Molecular Catalysis A: Chemical,2015,vol. 397,p. 150 - 157]
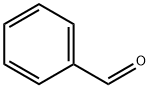
100-52-7
956 suppliers
$20.37/250g

104-94-9
490 suppliers
$9.00/1g

7472-54-0
62 suppliers
$12.00/1mg