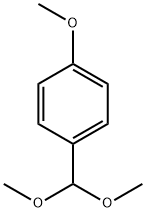
P-ANISALDEHYDE DIMETHYL ACETAL synthesis
- Product Name:P-ANISALDEHYDE DIMETHYL ACETAL
- CAS Number:2186-92-7
- Molecular formula:C10H14O3
- Molecular Weight:182.22
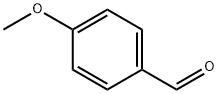
123-11-5
878 suppliers
$10.00/10g
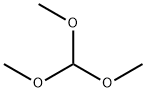
149-73-5
547 suppliers
$12.00/5g

2186-92-7
236 suppliers
$10.00/5g
Yield:2186-92-7 99%
Reaction Conditions:
with copper(II) tetrafluoroborate monohydrate in methanol at 20; for 1 h;Inert atmosphere;
Steps:
1 Synthesis of compound [17]
Synthesis of compound [17]
Under a nitrogen atmosphere, p-methoxybenzaldehyde [7] (2.7 g, 20 mmol) and trimethyl orthoformate (4.4 mL, 40 mmol) were dissolved in 8 mL of dry methanol, and to the solution, copper(II) tetrafluoroborate hydrate (48 mg) was added. The mixture was stirred at room temperature for 1 hour, and saturated sodium hydrogen carbonate was added to stop the reaction. To the solution, ethyl acetate was added to perform extraction and the organic phase was then washed with saturated sodium chloride. The organic phase was dried over sodium sulfate, then the sodium sulfate was filtered off, and the filtrate was concentrated under reduced pressure: Yield 99%; 1H NMR (500 MHz, CDC3): δ 7.36 (d, 2H, J=8.8 Hz), 6.89 (d, 2H, J=8.8 Hz), 5.35 (s, 1H), 3.81 (s, 3H), 3.31 (s, 6H).
References:
Ono, Fumiyasu;Shinkai, Seiji;Hirata, Osamu US2015/25157, 2015, A1 Location in patent:Paragraph 0144; 0145; 0146; 0147
67-56-1
776 suppliers
$7.29/5ml-f

123-11-5
878 suppliers
$10.00/10g

2186-92-7
236 suppliers
$10.00/5g
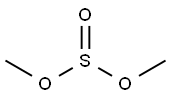
616-42-2
195 suppliers
$12.00/5g

123-11-5
878 suppliers
$10.00/10g

2186-92-7
236 suppliers
$10.00/5g
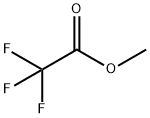
431-47-0
289 suppliers
$10.00/5g
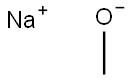
124-41-4
716 suppliers
$12.00/25g

123-11-5
878 suppliers
$10.00/10g

2186-92-7
236 suppliers
$10.00/5g
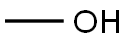
67-56-1
776 suppliers
$7.29/5ml-f
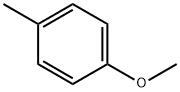
104-93-8
399 suppliers
$6.00/25g

2186-92-7
236 suppliers
$10.00/5g