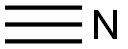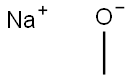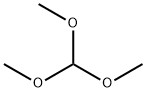
Trimethoxymethane synthesis
- Product Name:Trimethoxymethane
- CAS Number:149-73-5
- Molecular formula:C4H10O3
- Molecular Weight:106.12
HCN + 3 HOCH3 → HC(OCH3)3 + NH3
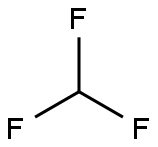
Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis.
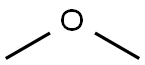
115-10-6
101 suppliers
$75.00/25g
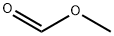
107-31-3
354 suppliers
$16.00/25mL

149-73-5
547 suppliers
$12.00/5g
Yield:149-73-5 90%
Reaction Conditions:
with boron trifluoride in Hexadecane at 110; for 12 h;Autoclave;
Steps:
2 Example 1. Preparation of triethyl orthobenzoate
General procedure: In a 50ml stainless steel autoclave with a magnetic stir bar, add 1.50g (10mmol) ethyl benzoate,In 0.1g n-hexadecane (internal standard) and 7.40g (100mmol) ether solution containing 0.068g (1mmol) boron trifluoride, heat up to 110 and react for 6.0 hours. After the reaction is over, cool down to 0-5 .The sample was analyzed by gas phase, and the conversion rate of ethyl benzoate was 98%, and the yield of triethyl orthobenzoate was 95%.
References:
CN111470954,2020,A Location in patent:Paragraph 0021; 0022
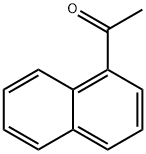
941-98-0
379 suppliers
$5.00/10g
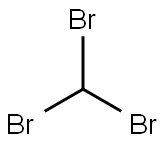
75-25-2
345 suppliers
$16.00/50g
2459-24-7
96 suppliers
$33.00/5g

149-73-5
547 suppliers
$12.00/5g
100-06-1
610 suppliers
$18.19/25g

75-25-2
345 suppliers
$16.00/50g
121-98-2
293 suppliers
$12.00/1mg

149-73-5
547 suppliers
$12.00/5g