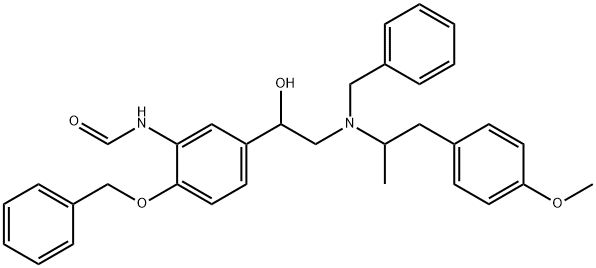
N,O-Dibenzylated formoterol synthesis
- Product Name:N,O-Dibenzylated formoterol
- CAS Number:43229-70-5
- Molecular formula:C33H36N2O4
- Molecular Weight:524.65
![RAC-N-BENZYL-N-[2-HYDROXYL-2-(4-BENZYLOXY-3-AMINOPHENYL)-ETHYL]-3-(4-METHOXYPHENYL)-2-PROPYLAMINE-D6](/CAS/20140608/GIF/CB1507768.gif)
43229-68-1
23 suppliers
inquiry

43229-70-5
98 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium carbonate in methanol;water
Steps:
14.d EXAMPLE 14
d. In 20 ml. of 5: 3 acetic anhydride-formic acid there was dissolved 5.5 g. of 3-amino-4-benzyloxy-α-[N-benzyl-N-(1-methyl-2-p-methoxyphenylethyl)aminomethyl]benzyl alcohol and after allowing the solution to stand overnight at room temperature, the solution was concentrated under reduced pressure. The residue was mixed with 50 ml. of methanol, 3 ml. of water, and 3.5 g. of sodium carbonate and the mixture was stirred for 2 hours at room temperature. Then, methanol was distilled off from the reaction product under reduced pressure and the residue formed was extracted with benzene. The extract was washed with water, dried over anhydrous magnesium sulfate, and then the solvent was distilled off to provide 5.1 g. of a crystalline powder of 4-benzyloxy-3-formylamino-α-[N-benzyl-N-(1-methyl-2-p-methoxyphenylethyl)aminomethyl] benzyl alcohol.
References:
Yamanouchi Pharmaceutical Co., Ltd. US3994974, 1976, A