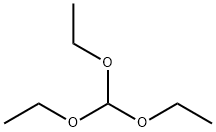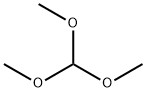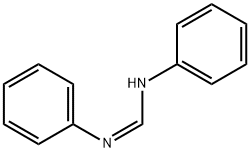
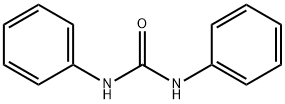
N,N'-diphenylformamidine synthesis
- Product Name:N,N'-diphenylformamidine
- CAS Number:864131-95-3
- Molecular formula:C13H12N2
- Molecular Weight:196.25
102-07-8
292 suppliers
$6.00/25g

864131-95-3
1 suppliers
inquiry
Yield:864131-95-3 98%
Reaction Conditions:
with iron(II) acetylacetonate;phenylsilane;tris(2-diphenylphosphinoethyl)phosphine in tetrahydrofuran at 100; for 24 h;Inert atmosphere;Glovebox;Schlenk technique;Reagent/catalyst;
Steps:
General procedure: The hydrosilylation reaction of the ureas of formula (II) to give formamidines of formula (I) is carried out according to the following experimental protocol.
The urea of formula (I) (1 equivalent), the catalyst (from 0.001 to 1 equivalent), the silane (1 to 3 equivalents) and the solvent are introduced, under an inert atmosphere, in a glove box, into a Schlenk tube which is subsequently sealed by a J. Young tap. The concentration of urea and of silane in the reaction mixture is approximately 0.5 mol·l-1 (concentration calculated on the basis of the volume of solvent introduced). The Schlenk tube is subsequently heated at a temperature of 100° C. until the urea has completely converted (reaction for 24 hours). Once the reaction is complete, the mixture is acidified with a 1N aqueous hydrochloric acid solution and the aqueous phase is washed 3 times with ether. Potassium hydroxide pellets are subsequently added to the aqueous phase up to basic pH and then extraction is carried out 3 times with ethyl acetate. After drying the organic phase over anhydrous magnesium sulfate, the ethyl acetate is evaporated under reduced pressure and the pure formamidine is obtained in the form of a white solid. In the event of the presence of other organic by-products, the formamidine can be purified by chromatography on silica gel. The use of a dichloromethane/methanol mixture as eluant makes it possible to obtain the analytically pure formamidine.
References:
US2015/266813,2015,A1 Location in patent:Paragraph 0109; 0110; 0114
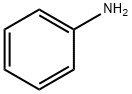
62-53-3
664 suppliers
$10.00/1g

864131-95-3
1 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

62-53-3
664 suppliers
$10.00/1g

864131-95-3
1 suppliers
inquiry
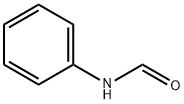
103-70-8
133 suppliers
$9.00/1g