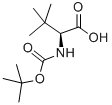
N-Boc-L-tert-Leucine synthesis
- Product Name:N-Boc-L-tert-Leucine
- CAS Number:62965-35-9
- Molecular formula:C11H21NO4
- Molecular Weight:231.29
20859-02-3
617 suppliers
$9.00/5G

24424-99-5
863 suppliers
$13.50/25G

62965-35-9
447 suppliers
$6.00/5g
Yield:62965-35-9 100%
Reaction Conditions:
with triethylamine in methanol at 0 - 5;
Steps:
1.A STEP A; 2(S)-tert-Butoxycarbonylamino-3,3-dimethyl-butyric Acid
STEP A: 2(S)-tert-Butoxycarbonylamino-3,3-dimethyl-butyric Acid To a suspension of L-tert-leucine (26.1 g, 0.2 mol) in methanol (150 ML) was added triethylamine (56 ML, 0.4 mol) and the mixture cooled to 0° C. To the mixture was added slowly a solution of di-tert-butyldicarbamate (48 g, 0.22 mol) in methanol (40 ML) such that an internal temperature of between 0 and 5° C. was maintained.. The reaction was allowed to stir overnight and the solvents were removed in vacuo.. The residue was dissolved in ethyl acetate (200 ML) and washed with 10% w/v aqueous citric acid solution (3*100 ML).. The organic layer was dried over MgSO4, filtered and the solvents removed in vacuo to give the title product as a pale yellow oil (48.9 g, >100%, residual solvent). 1H NMR (CDCl3): δ/ppm 9.20 (1H, bs), 5.10 (1H, m), 4.15 (1H, m), 1.45 (9H, s), 1.00 (9H, s).
References:
US6716878,2004,B1 Location in patent:Page column 14
26782-71-8
361 suppliers
$12.50/1G

24424-99-5
863 suppliers
$13.50/25G

62965-35-9
447 suppliers
$6.00/5g
153645-26-2
61 suppliers
$75.00/1g

62965-35-9
447 suppliers
$6.00/5g
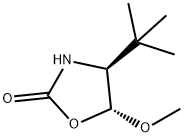
335627-81-1
2 suppliers
inquiry

62965-35-9
447 suppliers
$6.00/5g

20859-02-3
617 suppliers
$9.00/5G
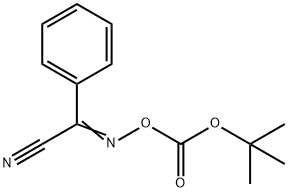
58632-95-4
276 suppliers
$7.00/5g

62965-35-9
447 suppliers
$6.00/5g