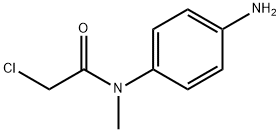
N-(4-aMinophenyl)-2-chloro-N-MethylacetaMide synthesis
- Product Name:N-(4-aMinophenyl)-2-chloro-N-MethylacetaMide
- CAS Number:855860-75-2
- Molecular formula:C9H11ClN2O
- Molecular Weight:198.65
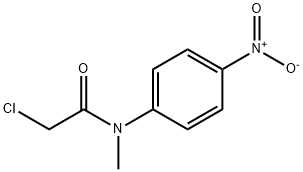
2653-16-9
133 suppliers
inquiry

855860-75-2
47 suppliers
inquiry
Yield:855860-75-2 85%
Reaction Conditions:
with iron(0);glacial acetic acid in water monomer at 20 - 45; for 1.5 h;
Steps:
Preparation of N-(4-aminophenyl)-2-chloro-N-methylacetamide (21):
To a solution of acetic acid (200 mL), 2-chloro-N-methyl-N-(4-nitrophenyl)acetamide[11] ( 50g, 0.218 mol) water (100 mL) and Iron-powder (61 g, 1.09 mol) were added at 20-30°C over a period of 30 minutes. Reaction mass temperature was raised to 40-45°C and stirred for 1h. After completion of reaction, the reaction mass was quenched into water (400 mL) & ethylacetate (500 mL) and stirred for 30 minutes. Filtered the Iron powder and cake was washed with ethylacetate (50 mL). The organic layer separated, washed with brine solution and dried over sodium sulfate. The organic layer was distilled off to get the crude amino compound 21, which was isolated in hexane as a solid (Wt. 38 g, Yield 85%). m.p.:83-86°C; IR (KBr, cm-1): 3436, 3354, 1655, 1518, 1121; 1H-NMR (400 MHz, DMSO): δ 3.10 (s, 3H), 3.93 (s, 2H), 5.7-6.0 (broad, 2H), 6.65 (d, 2H, J = 8.12 Hz), 7.01 (d, 2H, J = 7.64 Hz); 13C-NMR (100 MHz, DMSO): δ 37.62, 42.56, 115.62, 128.18, 131.94, 147.62 and 166.07; MS: m/z 199 [M]+1 and 201 [M]+2.
References:
Arava, Veerareddy;Gogireddy, Surendrareddy [Synthetic Communications,2017,vol. 47,# 10,p. 975 - 981] Location in patent:supporting information