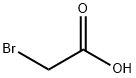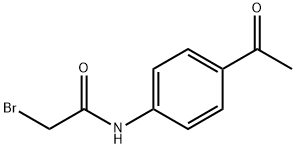
N-(4-ACETYLPHENYL)-2-BROMOACETAMIDE synthesis
- Product Name:N-(4-ACETYLPHENYL)-2-BROMOACETAMIDE
- CAS Number:29182-93-2
- Molecular formula:C10H10BrNO2
- Molecular Weight:256.1
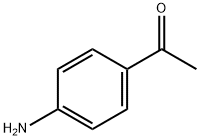
99-92-3
517 suppliers
$6.00/10g
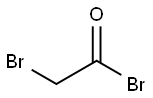
598-21-0
311 suppliers
$10.00/10g

29182-93-2
12 suppliers
$57.50/250mg
Yield:29182-93-2 95%
Reaction Conditions:
with potassium carbonate in dichloromethane;water at 0 - 20;
Steps:
4.1.2. Synthesis of N-(4-acetylphenyl)-2-bromoacetamide 5
To a stirred mixture of p-aminoacetophenone (6.30 mmol) in dichloromethane (20 ml) and potassium carbonate (1.30 mmol) in 100 ml water cooled in an ice bath, bromoacetyl bromide (1.86 g, 9.20 mmol) in 30 ml dichloromethane was added in a dropwise manner with stirring over 30 min. Stirring was continued for 2 h at 0 °C, then at room temperature overnight. The reaction mixture was extracted with (2 x 50 ml) dichloromethane and the organic layer was washed with distilled water (2 x 50 ml), dried over anhydrous sodium sulfate, filtered, evaporated on a rotary evaporator and the residue was recrystallized from 95% ethanol to give 1.53 g (95%) of compound 5, m.p. 155-156 °C.
References:
Abuo-Rahma, Gamal El-Din A.A.;Abdel-Aziz, Mohamed;Beshr, Eman A.M.;Ali, Taha F.S. [European Journal of Medicinal Chemistry,2014,vol. 71,p. 185 - 198]
62-53-3
659 suppliers
$10.00/1g

29182-93-2
12 suppliers
$57.50/250mg
22118-09-8
173 suppliers
$21.21/10gm:

99-92-3
517 suppliers
$6.00/10g

29182-93-2
12 suppliers
$57.50/250mg