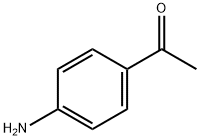
4-Aminoacetophenone synthesis
- Product Name:4-Aminoacetophenone
- CAS Number:99-92-3
- Molecular formula:C8H9NO
- Molecular Weight:135.16
the synthesis of a 4-Aminoacetophenone is include:
(1) parahydroxyacet-ophenone and 2-halogen-2-methyl propanamide reacts under alkaline condition and generates 2-(4-acetyl phenoxy group)-2-methyl propanamide;
(2) 2-(4-acetyl phenoxy group)-2-methyl propanamide generates N-(4-acetyl phenyl)-2-hydroxy-2-methyl propionic acid amide through the Smiles rearrangement reaction under alkaline condition;
(3) N-(4-acetyl phenyl)-2-hydroxy-2-methyl propionic acid amide generates para-aminoacetophenone through the heating hydrolysis reaction under alkaline condition.
Yield:99-92-3 95%
Reaction Conditions:
with hydrogen in glycerol at 100; for 2 h;
Steps:
2.2. Pd-catalysed hydrogenation
General procedure: 1 mmol of alkene (146 mg for (E)-4-phenyl-but-3-en-2-one;146 mg for coumarin; 165 mg for 4-nitroacetophenone; 180 mg fortrans-stilbene) was added to a 1 mL of solution of preformed nanoparticlesin glycerol (0.01 mmol Pd, 1.0 mol%). The resulting mixture was stirred at room temperature under argon in a Fisher-Porter bottle. The system was then pressurized with dihydrogen (3 bar) and stirred at100 °C for 3 h. The mixture was then cooled to room temperature. The catalytic mixture was extracted with dichloromethane (3 × 10 mL) and the combined organic phases were dried over anhydrous Na2SO4,filtered and the solvent evaporated under reduced pressure.
References:
Chahdoura, Faouzi;Favier, Isabelle;Pradel, Christian;Mallet-Ladeira, Sonia;Gómez, Montserrat [Catalysis Communications,2015,vol. 63,p. 47 - 51]
20062-24-2
25 suppliers
inquiry

99-92-3
530 suppliers
$6.00/10g
14235-81-5
266 suppliers
$22.39/1G

99-92-3
530 suppliers
$6.00/10g
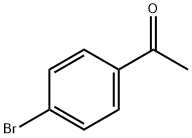
99-90-1
482 suppliers
$6.00/25g

99-92-3
530 suppliers
$6.00/10g
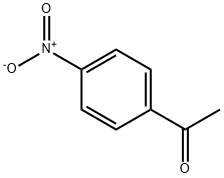
100-19-6
312 suppliers
$5.00/5g

99-92-3
530 suppliers
$6.00/10g