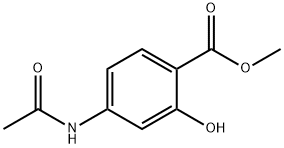
METHYL 4-ACETAMIDO-2-HYDROXYBENZOATE synthesis
- Product Name:METHYL 4-ACETAMIDO-2-HYDROXYBENZOATE
- CAS Number:4093-28-1
- Molecular formula:C10H11NO4
- Molecular Weight:209.2
Yield: 99%
Reaction Conditions:
with sodium hydrogencarbonate in water;ethyl acetate at 0 - 20; for 2.25 h;
Steps:
1.ii Step (ii)
Preparation of Methyl 4-acetylamino-2-hydroxy benzoate
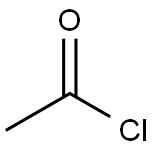
A solution of Methyl 4-amino-2-hydroxy benzoate (50.7 grams, 303.6 mmol, obtained in above step) in ethyl acetate (750 mL) was added to a stirred solution of water (250 mL) and sodium bicarbonate (34.9 grams, 415.5 mmol) cooled at 0 °C followed by acetyl chloride (29.7 mL, 415.5 mmol) over a period of 15 minutes. The reaction mixture was gradually warmed to room temperature and stirred for 2 hours. The two layers were separated and the organic layer was washed with brine, dried over anhydrous sodium sulphate and the solvent was removed under reduced pressure to obtain methyl 4-acetylamino-2-hydroxy benzoate (63.5 grams).Yield: 99 %.? - NMR (CDC13): δ 10.86 (bs, 1H), 7.78 (d, J = 8.6 Hz, 1H), 7.23 (s, 1H), 7.16 (bs, 1H), 7.10 (d, J = 8.6 Hz, 1H), 6.13 (bs, 1H), 3.92 (s, 3H), 2.19 (s, 3H);Mass (m/z): 208 (M-H)+.
References:
SUVEN LIFE SCIENCES LIMITED;NIROGI, Ramakrishna;MOHAMMED, Abdul Rasheed;YARLGADDA, Suresh;RAVELLA, Srinivasa Rao;SHINDE, Anil Karbhari;KAMBHAMPATI, Ramasastri;ROAYALLEY, Praveen Kumar;JAYARAJAN, Pradeep;BHYRAPUNENI, Gopinadh;PATNALA, Sriramachandra Murthy;RAVULA, Jyothsna;JASTI, Venkateswarlu WO2013/42135, 2013, A1 Location in patent:Page/Page column 11
127-09-3
1110 suppliers
$8.00/1 ea
4136-97-4
189 suppliers
$6.00/5g

4093-28-1
149 suppliers
$6.00/5g

4136-97-4
189 suppliers
$6.00/5g

4093-28-1
149 suppliers
$6.00/5g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4093-28-1
149 suppliers
$6.00/5g