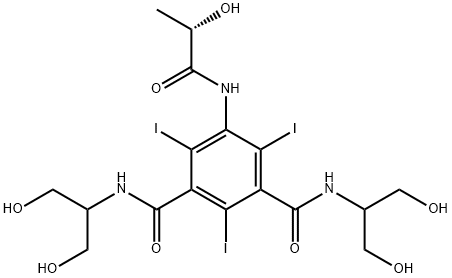
Iopamidol synthesis
- Product Name:Iopamidol
- CAS Number:60166-93-0
- Molecular formula:C17H22I3N3O8
- Molecular Weight:777.09
![(S)-5-[2-(acetyloxy)propanamido]-2,4,6-triiodo-1,3-di(chloroformyl)benzene](/CAS/20200119/GIF/60166-91-8.gif)
60166-91-8
4 suppliers
inquiry
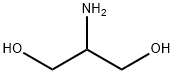
534-03-2
473 suppliers
$7.00/10g

60166-93-0
201 suppliers
$59.00/25mg
Yield:60166-93-0 94%
Reaction Conditions:
in ISOPROPYLAMIDE at 8 - 20; for 10 h;
Steps:
8
680 g of S-(-)-5-((2-acetyloxy)-1-oxopropyl)amino)-2,4,6-triiodo-1,3-benzendicarboxylic acid dichloride (prepared as described in WO 96/37460) are dissolved in 1360 g of dimethylacetamide at room temperature and after cooling at 15°C [Solution A ]. 181 g of 2-ammino-1,3-propanediol purified with the method described in the previous Example 1 are dissolved in 1360 g of dimethylacetamide and added to the solution A in one hour at 8-15°C whilst stirring. The reaction is completed after ten hours at room temperature. The reaction mixture is concentrated at 100°C at 10 mbar until 98% of the solvent is distilled. 1700 g of water are added to the residue and the solution is purified using the method described in WO 97/30735.Yield on dry Iopamidol: 94% Contents of by-products determined with HPLC method (according to the method described in USP XXIII-NF, 1996, V°suppl.) = 0.11% The by-product N-[2-hydroxy-1-(hydroxymethyl)ethyl]-- N'-(2,3-dihydroxypropyl)-5-(2-hydroxy-1-oxopropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide, coming from isoserinol, is under the detection limit. No presence of other by-products derived from 3-amino-1,2-propanediol are detected (see as ref. Pharmeuropa, 6, 343-345, 19949.
References:
BRACCO IMAGING S.p.A. EP883597, 2005, B1 Location in patent:Page/Page column 6-7
618-88-2
505 suppliers
$13.00/25g

60166-93-0
201 suppliers
$59.00/25mg
267881-76-5
1 suppliers
inquiry

60166-93-0
201 suppliers
$59.00/25mg
![5-Amino-N,N'-bis[2-hydroxy-1-(hydroxymethyl) ethyl]-2,4,6-triiodobenzene-1,3-dicarboxamide](/CAS/20180808/GIF/60166-98-5.gif)
60166-98-5
54 suppliers
$125.00/5mg

60166-93-0
201 suppliers
$59.00/25mg
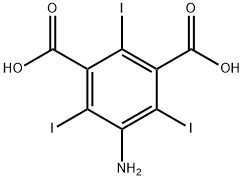
35453-19-1
349 suppliers
$6.00/5g

60166-93-0
201 suppliers
$59.00/25mg