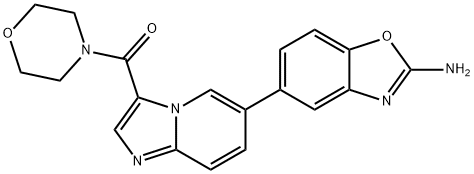
INK1117 synthesis
- Product Name:INK1117
- CAS Number:1268454-23-4
- Molecular formula:C19H17N5O3
- Molecular Weight:363.37
![(2-Aminobenzo[d]oxazol-5-yl)-boronic acid hydrochloride](/CAS/GIF/1404480-15-4.gif)
1404480-15-4
20 suppliers
inquiry
![Methanone, (6-?bromoimidazo[1,?2-?a]?pyridin-?3-?yl)?-?4-?morpholinyl-](/CAS/20200611/GIF/1434089-03-8.gif)
1434089-03-8
2 suppliers
inquiry

1268454-23-4
86 suppliers
$25.00/1mg
Yield: 85%
Reaction Conditions:
Stage #1:2-amino-benzooxazole-5-boronic acid hydrochloride;C12H12BrN3O2 in 1,4-dioxane;water at 20; for 0.166667 h;
Stage #2: with tetrakis(triphenylphosphine) palladium(0);sodium carbonate in 1,4-dioxane;water at 20 - 102;Inert atmosphere;Reflux;
Steps:
6 Preparation of (6-(2-aminobenzo [d] oxazol-5 -yl)imidazo [ 1 ,2-a]pyridin-3 - yl)(morpholino)methanone (Formula I)
[00342] Example6[00343] Formula I[00344] Preparation of (6-(2-aminobenzo [d] oxazol-5 -yl)imidazo [ 1 ,2-a]pyridin-3 - yl)(morpholino)methanone (Formula I):[00345] 1. A 5 L three-neck round bottom flask was equipped with a mechanical stirrer, thermocouple probe, Nitrogen / vacuum inlet and reflux condenser and placed into a heating mantle.[00346] 2. 1,4-Dioxane (3.75 L) and water (1.25 L) were added at room temperature.[00347] 3. Compound 9 (250 g) and Compound 3 (210 g) were added to the flask at room temperature.[00348] 4. The reaction mixture was stirred for 10 min at room temperature.[00349] 5. Sodium carbonate (300 g) was added to the flask followed byPd(PPh3)4 (47 g) undemitrogen at room temperature.[00350] 6. With stirring, the reactor was sparged with nitrogen for -30 minutes at [00351] 7. The reaction mixture was deoxygenated by performing 5 to 6 vacuum / nitrogen cycles.[00352] 8. The reaction mixture was heated to reflux (88°C to 102°C) under slight nitrogen bubbling and stirredfor 5 to 8 hours.[00353] 9. The reaction was monitored by HPLC.[00354] 10. Upon completion, the reaction mixture was cooled to 75 - 80°C.[00355] 11. Water (3.75 L) and ethyl acetate (1.25 L) were added to the reaction mixture at 75 - 80°C.[00356] 12. The resulting mixture was cooled to room temperature and stirred for 2 hours at room temperature.[00357] 13. The mixture was filtered and the solid collected was washed with water (2x 1.25 L), methanol (1.25 L) andethyl acetate (2 x 1.25 L).[00358] 14. The solidwas suspended in ethyl acetate (1.5 L) at room temperature for 1 hour.[00359] 15. The suspension was filtered and the solidcollected was washed with ethyl acetate (250 mL).[00360] 16. This process was repeated two more times.[00361] 17. The solidobtained was dried under vacuum to give a crude product (250 g, 85% yield; HPLC purity 98.1%; Pd level 1831 ppm).[00362] Further purification of the crude product:[00363] 18. The crude product (250g) was suspended in methanol (2.5 L) and HCl (158 mL) at room temperature.[00364] 19. The mixture was heated to 40 to 45°C to give a slightly cloudy solution.[00365] 20. Charcoal (250 g) was added to the reaction mixture at 40 to 45°C. [00366] 21. The resulting mixture was stirred for 30 minutes at 40 to 45 °C.[00367] 22. The hot solution was filtered through a poly pad with 2 inch celite bed and the cake was washed withmethanol (3 x 500 mL).[00368] 23. The solution was recharged to the flask (Pd level: 330 ppm).[00369] 24. The solution was heated to 40 to 45°C.[00370] 25. Charcoal (50 g) and silica thiol (50 g) were added at 40 to 45°C.[00371] 26. The mixture was stirred for 30 minutes at 40 to 45°C.[00372] 27. The hot solution was filtered through a poly pad and the cake was washed with methanol (250 mL)[00373] 28. This process was repeated one more time.[00374] 29. The methanol was removed under vacuum at 30 - 35°C to ~2.5 L.[00375] 30. The solution was cooled to 10 to 15°C.[00376] 31. Concentrated aqueous ammonia solution (200 mL) was added until reaching pH 8-9.[00377] 32. The reaction mixture was cooled to 0 to 5°C and stirred for 1 to 2 hours at 0 to 5°C.[00378] 33. The mixture was filtered and the cake was washed with water (2 x 500 mL) and methanol (2 x 500mL).[00379] 34. The solid collected was transfered back into a round bottom flask and suspended in ethyl acetate (2.5 L) at roomtemperature for 2 to 3 hours.[00380] 35. The solid was collected by filtration and washed with ethyl acetate (750 mL).36. The solid was dried under vacuum at ~50°C to constant weight to give thecompound of Formila I as an off-white solid (180 g, 61% yield; HPLC purity 98.5%; 'HNMR (DMSO-d6, 300 MHz) ? 9.1 (s, 1H), 8.1 (s, 1H), 7.8-7.65 (2H), 7.60-7.40 (m, 4H), 7.3-7.2 (1H), 3.8-3.6 (m, 8H); Pd level lppm).
References:
INTELLIKINE, LLC;MARTIN, Michael;WORRALL, Christopher, Peter;GANCEDO, Susanna, Del Rio;REN, Pingda WO2013/71272, 2013, A1 Location in patent:Paragraph 00342-00380
![6-Bromoimidazo[1,2-a]pyridine-3-carboxylicacid](/CAS/GIF/944896-42-8.gif)
944896-42-8
113 suppliers
$87.00/5g

1268454-23-4
86 suppliers
$25.00/1mg
40925-68-6
260 suppliers
$6.00/1g

1268454-23-4
86 suppliers
$25.00/1mg
![5-BROMOBENZO[D]OXAZOL-2-AMINE](/CAS/GIF/64037-07-6.gif)
64037-07-6
118 suppliers
$7.00/1g

1268454-23-4
86 suppliers
$25.00/1mg
![IMidazo[1,2-a]pyridine-3-carboxylic acid, 6-broMo-, ethyl ester](/CAS/GIF/372198-69-1.gif)
372198-69-1
115 suppliers
$14.00/250mg

1268454-23-4
86 suppliers
$25.00/1mg