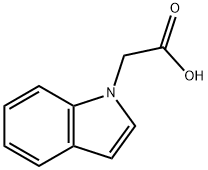
Indol-1-yl-acetic acid synthesis
- Product Name:Indol-1-yl-acetic acid
- CAS Number:24297-59-4
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
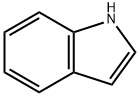
120-72-9
652 suppliers
$6.00/25g
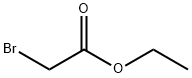
105-36-2
356 suppliers
$5.00/5 g

24297-59-4
122 suppliers
$5.00/250mg
Yield: 92%
Reaction Conditions:
with sodium hydroxide;tetrabutylammomium bromide in water;benzene
Steps:
1 (1H-Indol-1-yl)acetic Acid
EXAMPLE 1 (1H-Indol-1-yl)acetic Acid To a solution of ethyl 2-bromoacetate (25 g, 0.15 mole) and indole (11.7 g, 0.1 mole) in 100 ml of benzene is added a solution of sodium hydroxide (25 g, 0.626 mole) in 50 ml of water. To the obtained mixture is added tetra-n-butylammonium bromide (1.6 g, 0.5 mmole). The mixture is stirred at 25° C. for 16 hours. The aqueous phase is acidified to pH 3. The organic phase is separated, dried and evaporated to give the title compound in 92% yield. NMR (DMSO-D6) ppm (δ) 5.05 (s,2); 6.50 (d, 1), 6.9- 7.8 (m, 5).
References:
Yeda Research and Development Co. Ltd. US4139703, 1979, A
61155-69-9
5 suppliers
inquiry

24297-59-4
122 suppliers
$5.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24297-59-4
122 suppliers
$5.00/250mg
33140-80-6
17 suppliers
inquiry

24297-59-4
122 suppliers
$5.00/250mg

120-72-9
652 suppliers
$6.00/25g

24297-59-4
122 suppliers
$5.00/250mg