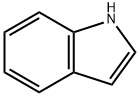
Indole synthesis
- Product Name:Indole
- CAS Number:120-72-9
- Molecular formula:C8H7N
- Molecular Weight:117.15
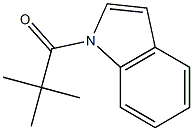
70957-04-9
3 suppliers
inquiry

120-72-9
652 suppliers
$6.00/25g
Yield:120-72-9 100%
Reaction Conditions:
with n-butyllithium;diisopropylamine in tetrahydrofuran;hexanes at -78 - 45; for 2 h;Inert atmosphere;
Steps:
4.3. General procedures for the deprotection of N-pivaloylindoles
General procedure: Method A: To 1.6 M solution of butyllithium in hexanes (2 equiv) was added dropwise a stirred solution of diisopropylamine (2 equiv) in dry THF (2 mL×mmol) at 0 °C, under an argon atmosphere. Stirring was continued for 10 min at 0 °C, and the solution of LDA thus prepared was added via cannula to a stirred solution of the suitable 1-pivaloyl derivative (1 equiv) in THF (2 mL×mmol), under an argon atmosphere at -78 °C. When addition was complete, the reaction mixture was heated in an oil bath at 40-45 °C for 2 h, cooled and poured onto a saturated aqueous NH4Cl solution (30 mL), which was then extracted with CH2Cl2 (3×15 mL). The combined organic layers were dried over Na2SO4 and evaporated, and the residue was chromatographed on silica gel, eluting with 9:1 EtOAc-petroleum ether. Evaporation of the mobile phase yielded the deprotected indole, which was identical in all respects to the commercially available sample employed as starting material for the protection reaction.
References:
Ruiz, Míriam;Sánchez, J. Domingo;López-Alvarado, Pilar;Menéndez, J. Carlos [Tetrahedron,2012,vol. 68,# 2,p. 705 - 710] Location in patent:experimental part
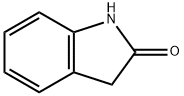
59-48-3
480 suppliers
$5.00/1g

120-72-9
652 suppliers
$6.00/25g
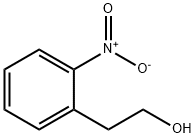
15121-84-3
126 suppliers
$7.00/1g

120-72-9
652 suppliers
$6.00/25g
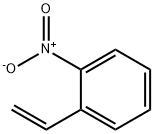
579-71-5
44 suppliers
inquiry

120-72-9
652 suppliers
$6.00/25g
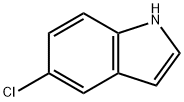
17422-32-1
377 suppliers
$6.00/1g

120-72-9
652 suppliers
$6.00/25g