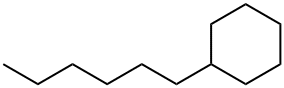
HEXYLCYCLOHEXANE synthesis
- Product Name:HEXYLCYCLOHEXANE
- CAS Number:4292-75-5
- Molecular formula:C12H24
- Molecular Weight:168.32
86-74-8
337 suppliers
$10.00/5g
92-51-3
132 suppliers
$21.00/25g

4292-75-5
39 suppliers
$128.00/10mL
827-52-1
298 suppliers
$5.00/10g
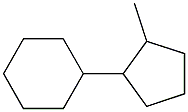
5405-90-3
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen in decane;para-xylene at 349.84; under 22502.3 Torr;Catalytic behavior;Flow reactor;Reagent/catalyst;Temperature;
Steps:
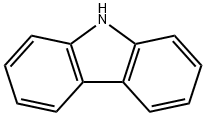
Carbazole HDN activity measurements
Carbazole HDN activity measurements Carbazole HDN activity measurements were carried out usinga fixed-bed, continuous flow reactor operating at a total pressure of 3.0 MPa and temperatures in the range of 548-673 K. Thereactor feed consisted of either 1000 ppm carbazole and 500 ppm dodecane in a 39.85 wt% p-xylene/decane solution or 3000 ppmbenzothiophene, 1000 ppm carbazole, and 500 ppm dodecane in a39.55 wt% p-xylene/decane solution. The 500 ppm dodecane servedas an internal standard for gas chromatographic analysis of thereactor effluent. The liquid feed (5.4 mL/h) was injected into a50 mL/min flow of hydrogen and vaporized prior to entry intothe reactor. 0.25 g of catalyst (16-20 mesh size) was diluted withquartz flakes to a total volume of 5 mL and loaded into a reactortube having a diameter of 1.1 cm and length of 40 cm. The internalreactor temperature was monitored with an axially mounted ther-mocouple in direct contact with the catalyst bed. Metal phosphideand bimetallic phosphide catalysts were pretreated by heatingfrom room temperature to 650 K in 1 h in a 60 mL/min flow ofH2and held at this temperature for 2 h, then cooled to roomtemperature in continued H2flow. A commercial Ni-Mo/Al2O3cat-alyst (Shell 424) was subjected to a sulfidation pretreatment byheating from room temperature to 650 K in 1 h in a 60 mL/minflow of 3.0 mol% H2S/H2mixture, held at this temperature for2 h, and then cooled to room temperature in continued H2S/H2flow. After pretreatment, with the catalyst samples at room temper-ature, the reactor was pressurized to 3.0 MPa with H2. The catalystbed was heated to 548 K over 15 min in a 50 mL/min flow of H2and liquid feed injection was begun once the reactor reached theoperating temperature. The reactor was allowed to stabilize underoperating conditions for 3 h prior to sampling reactor effluent at30 min intervals over 2.5 h. The catalyst temperature was raised25 K, the reactor stabilized for 3 h, followed by sampling of the reac-tor effluent at 30 min intervals. This procedure was repeated untilsampling at the maximum catalyst temperature (623-673 K) wascompleted. The first effluent sample collected at each temperaturewas discarded; the four subsequent effluent samples were analyzedoff-line using a gas chromatograph (Agilent 6890N) equipped withan HP-5 column and a flame ionization detector. HDN product iden-tification was conducted by GC/MS and the GC was calibrated usingserial diluted product standard solutions to allow product quan-tification of reactor effluent samples. Based on this method, carbonbalances of 90-95% were achieved, indicating that the analyses ofthe reactor effluent accounted for all of the major reaction prod-ucts. The majority of the HDN activity measurements were done induplicate to ensure reproducibility.
References:
Bowker, Richard H.;Ilic, Boris;Carrillo, Bo A.;Reynolds, Michael A.;Murray, Brendan D.;Bussell, Mark E. [Applied Catalysis A: General,2014,vol. 482,p. 221 - 230]
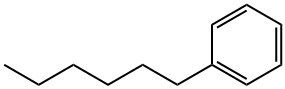
1077-16-3
157 suppliers
$19.30/1ml

4292-75-5
39 suppliers
$128.00/10mL

86-74-8
337 suppliers
$10.00/5g

92-51-3
132 suppliers
$21.00/25g

4292-75-5
39 suppliers
$128.00/10mL

5405-90-3
0 suppliers
inquiry

86-74-8
337 suppliers
$10.00/5g
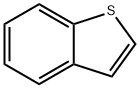
95-15-8
373 suppliers
$6.00/5g

92-51-3
132 suppliers
$21.00/25g

4292-75-5
39 suppliers
$128.00/10mL

827-52-1
298 suppliers
$5.00/10g

5405-90-3
0 suppliers
inquiry
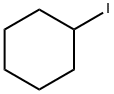
626-62-0
138 suppliers
$10.00/1g

3761-92-0
105 suppliers
$55.00/100ml

4292-75-5
39 suppliers
$128.00/10mL