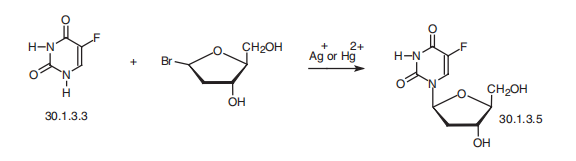
Floxuridine synthesis
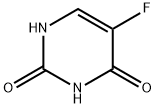
- Product Name:Floxuridine
- CAS Number:50-91-9
- Molecular formula:C9H11FN2O5
- Molecular Weight:246.19
1582-79-2
13 suppliers
$70.00/50mg

50-91-9
392 suppliers
$6.00/250mg
Yield:50-91-9 86.2%
Reaction Conditions:
with methanol;ammonia at 10 - 25;
Steps:
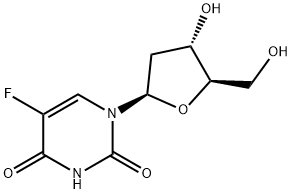
2 Example 2: Preparation of 2'-Deoxy-5-fluorouridine (FUDR).
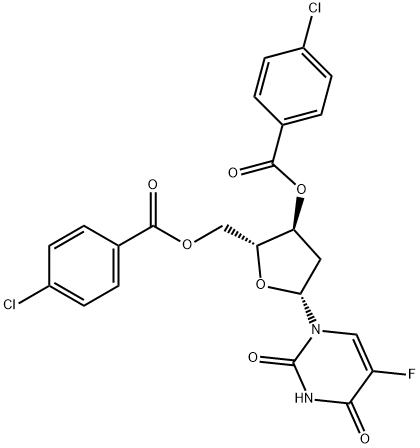
To a solution of methanolic ammonia (10-12% w/w; 500 mL, 10 v/w) at 10 °C was added 3',5'-di-0-(4-chlorobenzoyl)-5-fluoro-2'-β-deoxyuridine 3 (50 g, 0.0955 mole). The reaction mixture was raised to 25 °C over 1-2 h and maintained at this temperature for 20-24 h. When the reaction was complete, as determined by HPLC, it was filtered through Celite at 25 °C. The Celite was washed with MeOH (100 mL, 2 v/w) and the combined filtrate was concentrated to between 50-100 mL under vacuum (600 mmHg, Output: Wt. of the compound FUDR: 20.2 g Yield: 86.2% (HPLC Purity: 99.94% Characterisation data for FUDR is consistent with that which has been previously reported, e.g. in Aoyama et al. (Bull. Chem. Soc. Jpn., 1987, 60, 2073-2077).
References:
WO2019/53476,2019,A1 Location in patent:Page/Page column 33-34
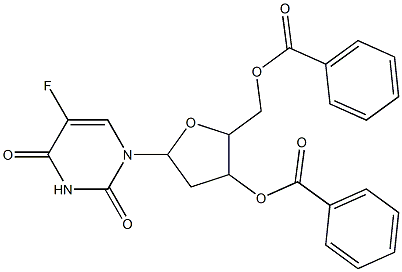
2691-71-6
0 suppliers
inquiry

50-91-9
392 suppliers
$6.00/250mg
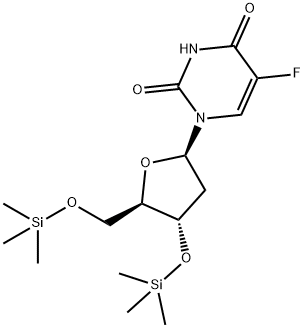
54202-96-9
0 suppliers
inquiry

50-91-9
392 suppliers
$6.00/250mg