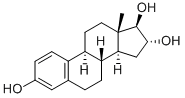
Estriol synthesis
- Product Name:Estriol
- CAS Number:50-27-1
- Molecular formula:C18H24O3
- Molecular Weight:288.38

![1,3,5[10]-ESTRATRIENE-3,16ALPHA-DIOL-17-ONE](/CAS/GIF/566-76-7.gif)
566-76-7
65 suppliers
$107.00/1mg

50-27-1
530 suppliers
$11.00/100mg
Yield:50-27-1 94%
Reaction Conditions:
with methanol;sodium tris(acetoxy)borohydride in 1,4-dioxane at 20 - 25;
Steps:
4
Example: 4: Reduction to 17p-estriol:Preparation of estra-l,3,5,(10)-trien-3,16a,17P -triol; To a 1 : 1 mixture of methanol: dioxane (500ml) in a one litre round bottomed flask, was added 3,16a -Hydroxy-estra-1,3,5 (10)-trien-17-one (22gm, 76.7mmole) and stirred to obtain homogeneous solution. At this point, sodium triacetoxyborohydride (16gm, 76mmole)was added slowly in lots so that the internal temperature does not exceed 20°C. The mixture was stirred for four hours at room temperature (23-25°C) after which another lot of reductant (25gm,l 17mmole)was added in four lots, each lot in one hour interval, after which the reaction mixture concentrated under reduced pressure and dil. HC1 (IN solution ,450ml) was added slowly to the residue and stirred for 10 minutes vigorously. The precipitated solid was filtered and washed with water (2 X 1 10ml) .The solid obtained was suspended in acetone (250ml) and stirred for 10 minutes and filtered to obtain the title compound.Yield : 20.5gm (94%).HPLC Purity : 99.53%]H NMR (400MHz,DMSO +CDC13)0.66(3H,s), 1.20-2.8(13H,m), 3.29(lH,m), 3.83(lH,m), 4.57(lH,dd,J=5Hz and 1.2Hz), 4.6(lH,dd,J=5Hz and 1.2Hz), 6.43(1 H,s), 6.50(lH,d,J=8Hz), 7.03(lH,d,J=8.4Hz)
References:
WO2012/32529,2012,A1 Location in patent:Page/Page column 10
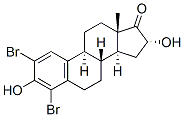
79258-14-3
15 suppliers
$80.00/5mg

50-27-1
530 suppliers
$11.00/100mg
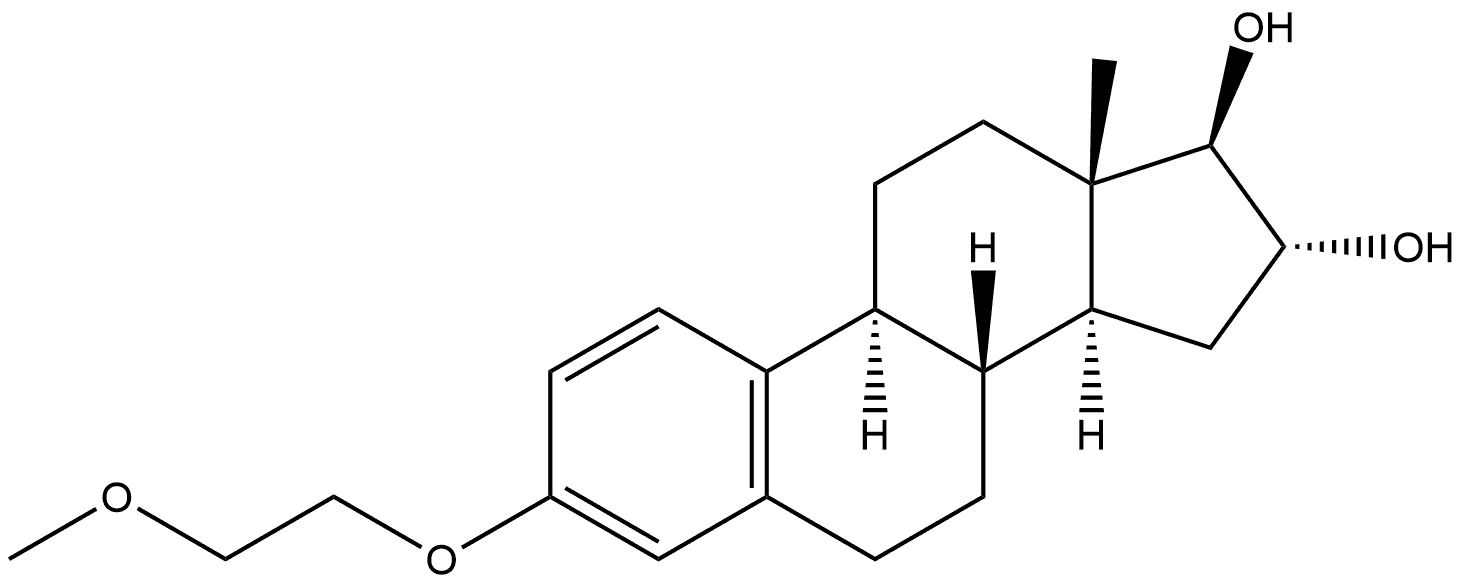
123715-88-8
0 suppliers
inquiry

50-27-1
530 suppliers
$11.00/100mg
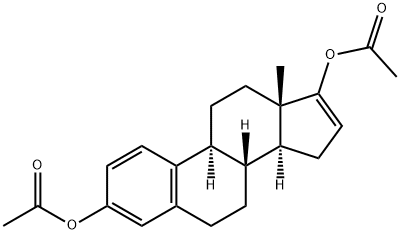
20592-42-1
19 suppliers
$185.00/250mg

50-27-1
530 suppliers
$11.00/100mg
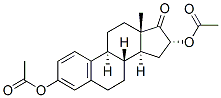
1247-71-8
3 suppliers
inquiry

50-27-1
530 suppliers
$11.00/100mg