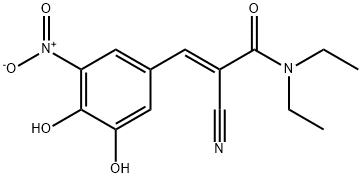
Entacapone synthesis
- Product Name:Entacapone
- CAS Number:130929-57-6
- Molecular formula:C14H15N3O5
- Molecular Weight:305.29
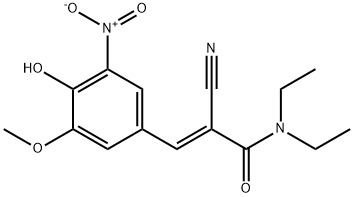
857629-78-8
44 suppliers
inquiry

130929-57-6
462 suppliers
$5.00/50mg
Yield:130929-57-6 98%
Reaction Conditions:
Stage #1: (2E)-2-cyano-3-(3-methoxy-4-hydroxy-5-nitro-phenyl)-N,Ndiethylprop-2-enamidewith aluminum (III) chloride;triethylamine in dichloromethane at 0 - 5; for 3 - 4 h;Heating / reflux;
Stage #2: with hydrogenchloride in dichloromethane at 0 - 20; for 0.5 h;
Steps:
2 Preparation of N,N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-acrylamide (entacapone); (I)
A round-bottom flask under nitrogen atmosphere is loaded with N,N-diethyl-2-cyano-3-(-3-methoxy-4-hydroxy-5-nitrophenyl-)-acrylamide (134 g, 0.421 mol) dissolved in dichloromethane (0.65 l) and triethylamine (128 g, 1.26 mol). The solution is cooled to 0-5° C. and aluminium trichloride (67.4 g, 0.505 mol) is added in portions. After that, the mixture is refluxed for 3-4 hours, until completion of the conversion. The reaction mixture is cooled to 0-5° C., a 20% hydrochloric acid solution (0.5 l) is dropped in the mixture, which is left under stirring at room temperature for 30 minutes. The resulting solid is filtered, washing with water (0.1 l×3), and dried in a static dryer under reduced pressure at 50° C.; 126 g are obtained. Yield: 98%.1H-NMR (300 MHz), δ (DMS0) 7.90 (d, 1H); 7.75 (d, 1H); 7.60 (s, 1H); 3.40 (m, 4H); 1.15 (m, 6H).
References:
US2008/146829,2008,A1 Location in patent:Page/Page column 4
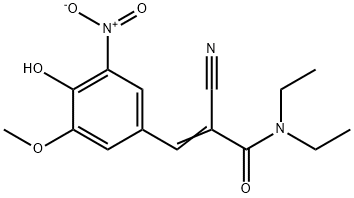
146698-91-1
18 suppliers
inquiry

130929-57-6
462 suppliers
$5.00/50mg
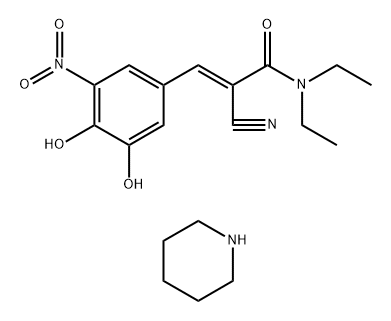
1047659-01-7
3 suppliers
inquiry

130929-57-6
462 suppliers
$5.00/50mg
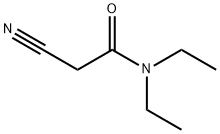
26391-06-0
218 suppliers
$11.00/1g
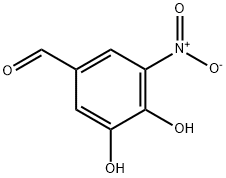
116313-85-0
325 suppliers
$5.00/1g

130929-57-6
462 suppliers
$5.00/50mg

26391-06-0
218 suppliers
$11.00/1g

116313-85-0
325 suppliers
$5.00/1g

130929-57-6
462 suppliers
$5.00/50mg
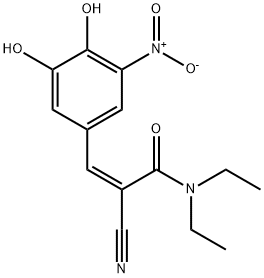
145195-63-7
94 suppliers
$118.00/1mg