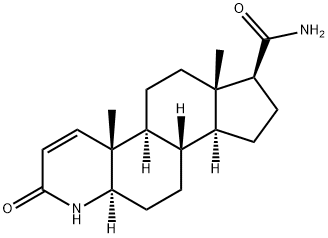
Dutasteride Related Impurity 1 synthesis
- Product Name:Dutasteride Related Impurity 1
- CAS Number:104214-61-1
- Molecular formula:C19H28N2O2
- Molecular Weight:316.44
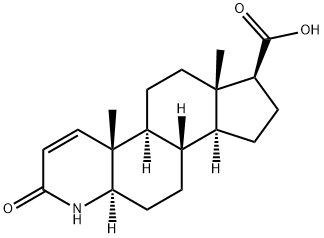
104239-97-6
197 suppliers
$56.00/2g

104214-61-1
28 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 3-Oxo-4-aza-5α-androst-1-ene-17β-carboxylic acidwith pyridine;thionyl chloride in toluene at 25 - 35; for 2.25 - 3.25 h;
Stage #2: with ammonia for 8 - 10 h;
Steps:
1 EXAMPLE 1; PREPARATION OF 3-OXO-4-AZA-5α-ANDROST-1-ENE-17β-CARBOXIMIDE (FORMULA III)
3-Oxo-4-aza-5α-androst-ene-17β-carboxylic acid (50 gram) and toluene (750 ml) were mixed together and heated at azeotropic reflux condition for 30 to 60 minutes. (Trace amount of water was removed azeotropically). The resulting solution was cooled to 25 to 35 degree C. under nitrogen atmosphere. Pyridine (6.3 ml) was added to the cooled solution, which was then stirred for about 15 minutes. Then, thionyl chloride (14.0 ml) was added slowly for over 20 minutes. The resulting reaction mixture was maintained at 25-35° C. temperature for about 2-3 hours and then ammonia gas was passed through the reaction mixture till the reaction was completed (8 to 10 hrs). After the completion of the reaction mixture was filtered and washed with toluene (100 ml). The resulting compound was dried for 1-2 hours. The resultant wet material was slurried in water (500 ml) for about 2 hours. Filtered the solid and washed with water (50.0 ml) to get the reaction mass pH up to 6.5 to 7.5. The filtered compound was dried at 70-75° C.
References:
US2005/59692,2005,A1 Location in patent:Page/Page column 3; 4
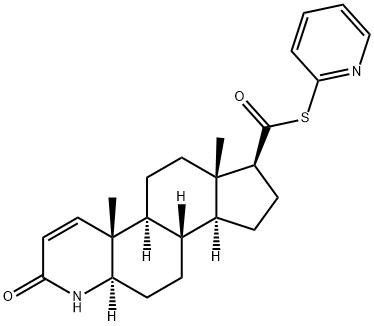
103335-49-5
3 suppliers
inquiry

104214-61-1
28 suppliers
inquiry
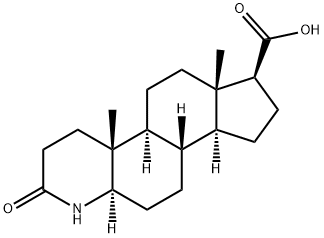
103335-55-3
202 suppliers
$40.00/1g

104214-61-1
28 suppliers
inquiry